P627 HLA DQA1*05 was not associated to primary non-response or loss of response to first anti-TNF in real world Inflammatory Bowel Disease patients.
Pascual Oliver, A.(1)*;Cuarán, C.(1);Casas-Deza, D.(1,2);García-López, S.(1,2);Corsino-Roche, P.(1,2);Sierra Moros, E.(1);Julián-Gomara, A.B.(1);Vicente-Lidón, R.(1);
(1)Hospital Universitario Miguel Servet, Gastroenterology, Zaragoza, Spain;(2)Instituto de Investigación Sanitaria de Aragón, Gastroenterology, Zaragoza, Spain;
Background
Biologics have been a breakthrough in the management of patients with inflammatory bowel disease (IBD). However, we lack predictors of response to these drugs. A recent study has shown a significant association between HLA DQA*105 mutations and the development of loss of response to anti-TNF mediated by immunogenicity. The high quality of the study and the wide differences found, have led to its quick practical implementation in the choice of biological agent. However, the supporting evidence is limited in clinical practice.
Methods
Retrospective cohort study, carried out at the Miguel Servet Hospital, Zaragoza, Spain. We included IBD patients who had received anti-TNF therapy as first biologic and whose HLA DQA1*05 had been determined. Primary non-response and secondary failure (assessed by survival analysis) has been evaluated as well as safety outcomes.
Results
199 IBD patients [161 (81%) Crohn´s disease (CD) 38 (19%) ulcerative colitis (UC)] were included. 42.4% had HLA DQA1*05 mutation and 60% received combination therapy with immunomodulators at the start of anti-TNF treatment. Median follow-up was 24 months (IQR 11-66). No statistically significant differences were found in primary non-response to anti-TNF (87,8% vs 12,2%, p = 0,825), depending on the presence or absence of HLA mutation. No differences in secondary loss of response according to HLA mutation in any of the analyses performed (full cohort, according to IBD or anti TNF type) were observed (Figure 1). Secondary loss of response has also been assessed according to the use or non-use of concomitant immunomodulator. Again, no differences were observed in this analysis (Figure 2). Multivariate analysis, like the univariate analyses, showed that the presence of the HLA-DQA1*05 mutation was not associated with a higher rate of secondary loss of response or shorter time to failure. In terms of safety, no significant differences were found in the rate of infusion reactions or serious adverse events between carrier and non-carrier patients.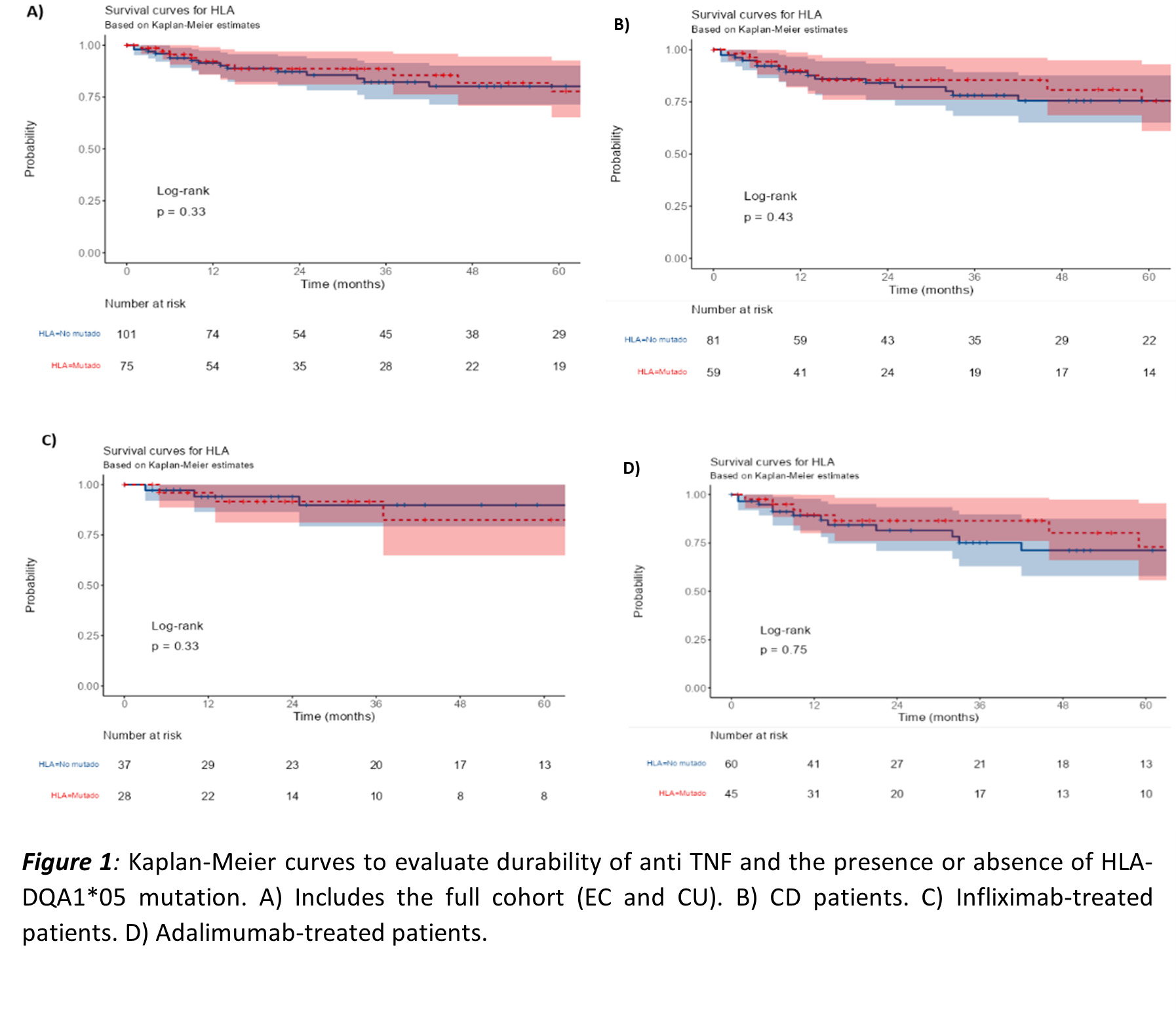

Conclusion
In our real-life cohort of IBD patients treated for the first time with anti-TNF, the presence of the HLA-DQA1*05 mutation did not act as a predictor of response failure, either primary or secondary. The safety of anti-TNF treatment has also not been influenced by the mutation. More data is needed to incorporate HLA DQA1*05 determination into routine clinical practice as a key factor in individual decision making.


