P632 Etrasimod induction therapy in moderately to severely active Crohn’s disease: results from a phase 2, randomised, double-blind substudy
D'Haens, G.(1)*;Dubinsky, M.C.(2);Peyrin-Biroulet, L.(3,4);Danese, S.(5);Sands, B.E.(6);Wolf, D.C.(7);Yarur, A.(8);Chiorean, M.(9);Dray, D.(10);Modesto, I.(11);Tan, H.(12);Gu, G.(13);Lopez, C.(14);Su, C.(15);Zhang, J.(16);McDonnell, A.(17);Schreiber, S.(18);Feagan, B.G.(19);Vermeire, S.(20);
(1)University of Amsterdam, Gastroenterology, Amsterdam, The Netherlands;(2)Feinstein IBD Center- Mount Sinai, Gastroenterology, New York- New York, United States;(3)University of Lorraine- Inserm- NGERE, Gastroenterology, Nancy, France;(4)Groupe Hospitalier privé Ambroise Paré - Hartmann- Paris IBD center, Gastroenterology, Neuilly sur Seine, France;(5)IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Gastroenterology, Milan, Italy;(6)Dr. Henry D. Janowitz Division of Gastroenterology- Icahn School of Medicine at Mount Sinai, Gastroenterology, New York- New York, United States;(7)Atlanta Gastroenterology Associates, Gastroenterology, Atlanta- Georgia, United States;(8)Cedars-Sinai Medical Center, Gastroenterology, Los Angeles- California, United States;(9)IBD Center- Swedish Medical Center, Gastroenterology, Seattle- Washington, United States;(10)Pfizer Inc, Global Medical Affairs- Gastroenterology, New York- New York, United States;(11)Pfizer Inc, Global Medical Affairs- Gastroenterology, Madrid, Spain;(12)Pfizer Inc, Early Internal/External Development, New York- New York, United States;(13)Pfizer Inc, Clinical Development & Operations- Inflammation & Immunology, New York- New York, United States;(14)Arena Pharmaceuticals- which was acquired by Pfizer in March 2022, Clinical Development, San Diego- California, United States;(15)Pfizer Inc, Global Product Development, New York- New York, United States;(16)Pfizer Inc, Biostatistics, New York- New York, United States;(17)Pfizer Ltd., Safety Risk, Walton Oaks- Surrey, United Kingdom;(18)University Hospital Schleswig-Holstein- Department Internal Medicine I- Kiel University, Internal Medicine, Kiel, Germany;(19)University of Western Ontario/Alimentiv Inc, Gastroenterology, London- Ontario, Canada;(20)University Hospitals Leuven, Gastroenterology, Leuven, Belgium;
Background
Etrasimod is an investigational, once-daily, oral, selective sphingosine 1-phosphate receptor 1,4,5 (S1P1,4,5) modulator in development to treat immune-mediated inflammatory disorders. CULTIVATE (NCT04173273) is a seamless phase 2/3 trial comprising 5 substudies designed to evaluate the efficacy, safety, and tolerability of etrasimod in subjects with moderately to severely active Crohn’s disease (CD). Here, we report the induction data from the first substudy (Substudy A).
Methods
Substudy A was a phase 2, randomised, double-blind study using a 14-week induction period followed by a 52-week extension period. Adults (18-80 years) with moderately to severely active CD and an inadequate response, loss of response to, or intolerance to ≥1 conventional or advanced treatments for CD were randomised 1:1 to once-daily etrasimod 2 mg or 3 mg. Subjects were stratified by baseline biologic failure status and oral corticosteroid use. The primary efficacy endpoint was achievement of endoscopic response (defined as endoscopic remission [SES-CD ≤4 and ≥2-point reduction from baseline with no subscore >1] or ≥50% decrease in SES-CD score) at week 14. Secondary/exploratory endpoints included achievement of endoscopic remission, clinical remission (CDAI <150), and IBDQ remission (IBDQ ≥170) at week 14. Outcomes were analysed in the Full Analysis Set (all randomised subjects treated with ≥1 dose) using NRI or observed data.
Results
Of 83 subjects randomised to etrasimod 2 mg or 3 mg, respectively, 32/42 (76.2%) and 36/41 (87.8%) completed week 14 (Table 1). Overall, 21.4% and 9.8% of subjects in the 2-mg and 3-mg groups, respectively, achieved the primary endpoint of endoscopic response, 14.3% and 7.3% achieved endoscopic remission, 31.0% and 43.9% achieved clinical remission, and 33.3% and 40.0% achieved IBDQ remission at week 14 (Figure 1). Most treatment-emergent AEs were mild or moderate, with more AEs reported in the 3-mg vs 2-mg group. The incidence of serious AEs (4.8% and 2.4%) and AEs leading to discontinuation (4.8% and 7.3%) was low in the 2-mg and 3-mg groups, respectively (Table 2). Two subjects in the 3-mg group experienced second degree atrioventricular block type 1 TEAEs (during 2-mg titration).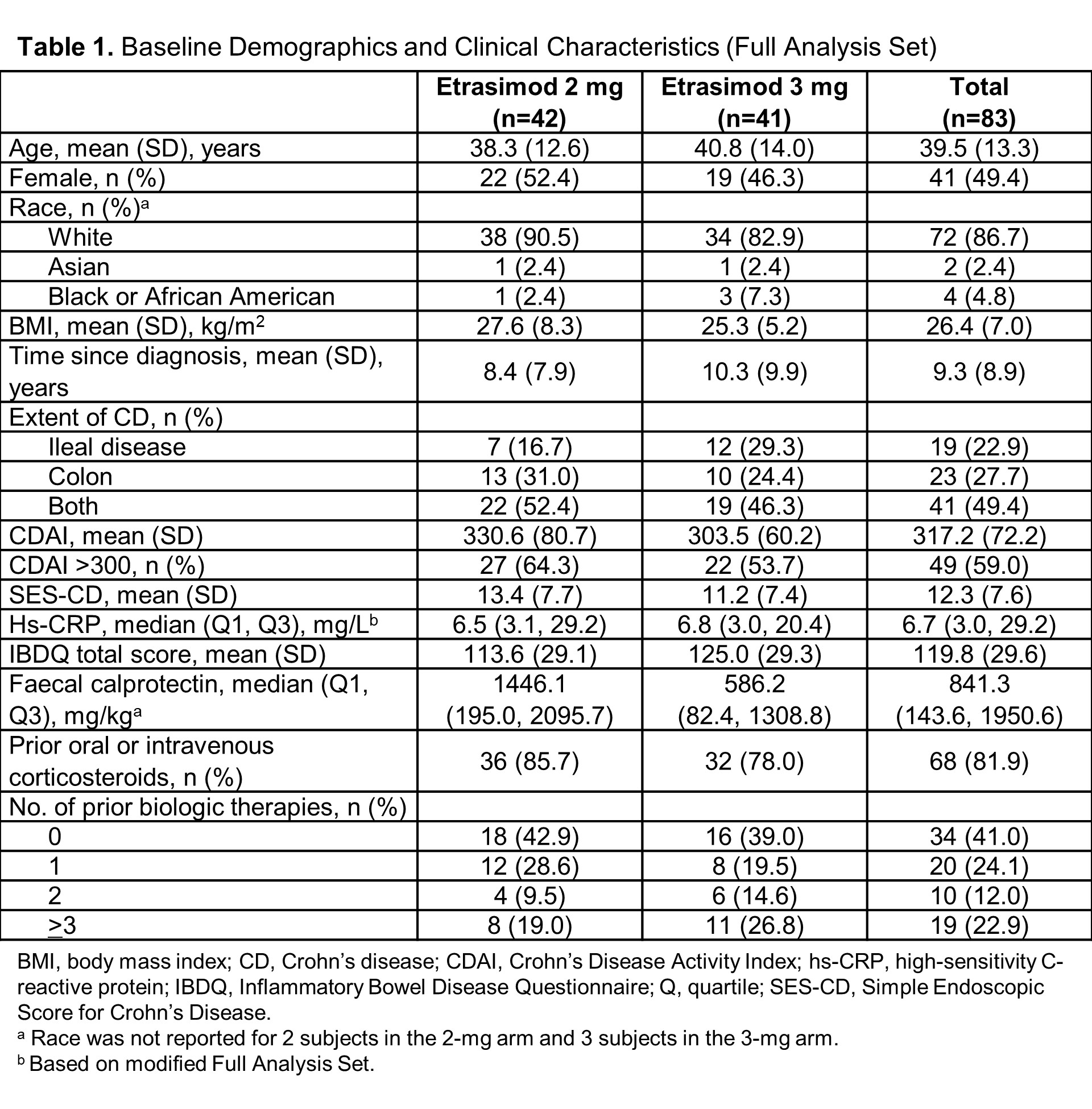


Conclusion
Data from this substudy of subjects with moderately to severely active CD suggest endoscopic and clinical improvement with both etrasimod 2 mg and 3 mg. However, the small sample size and lack of placebo arm limit the ability to draw conclusions about efficacy including comparison between the 2-mg vs 3-mg groups. Both doses were well tolerated and safety was consistent with other etrasimod clinical programmes. Data from the extension phase of Substudy A and the placebo-controlled, dose-ranging phase 2b Substudy 1 are forthcoming.


