P654 Controversies in the management of anti-TNF therapy in Crohn’s disease patients. A Delphi consensus
Gonzalez Lama, Y.(1)*;Ricart, E.(2);Carpio, D.(3);Bastida, G.(4);Ceballos, D.(5);Ginard, D.(6);Marín-Jimenéz, I.(7);Menchén, L.(8);Muñoz, F.(9);
(1)Hospital Universitario Puerta de Hierro- Majadahonda, Gastroenterology Department, Madrid, Spain;(2)Hospital Clínic de Barcelona, Inflammatory Bowel Disease Unit, Barcelona, Spain;(3)Complexo Hospitalario Universitario de Pontevedra, Gastroenterology Department, Pontevedra, Spain;(4)La Fe University and Polytechnic Hospital, Gastroenterology Department, Valencia, Spain;(5)Hospital Universitario Doctor Negrin, Gastroenterology Department, Las Palmas de Gran Canaria, Spain;(6)Hospital Universitario Son Espases, Gastroenterology Department, Palma de Mallorca, Spain;(7)Hospital Universitario Gregorio Marañón, Gastroenterology Department- Departamento de Medicina- Facultad de Medicina- Universidad Complutense de Madrid, Madrid, Spain;(8)Hospital General Universitario-Insitituto de Investigación Sanitaria Gregorio Marañón, Gastroenterology Department- Departamento de Medicina- Facultad de Medicina- Universidad Complutense de Madrid, Madrid, Spain;(9)Hospital Universitario de Salamanca, Gastroenterology Department, Salamanca, Spain;
Background
Over the last years significant advances have arisen in CD. New therapies with different mechanisms of action have been approved for the management of Crohn’s disease (CD). However, there is limited evidence on optimal positioning of agents as first-or second-line therapies due to the lack of head-to-head robust comparative data. Our aim was to establish feasible and practical recommendations for the management of patients with moderate to severe CD with the use of anti-tumour necrosis factor (TNF) drugs, with special focus on controversial areas.
Methods
A group of 9 expert gastroenterologists identified current relevant clinical controversies in the management of CD with anti-TNF therapies. A comprehensive literature review was performed to analyze them and a survey was launched to examine current clinical practice. This was a structured survey organized in 2 main sections that included several questions: 1) Sociodemographic and practice related questions; 2) Opinion and attitude in daily practice related to: the use of anti-TNFs, vedolizumab or ustekinumab in naïve and refractory patients, the role of HLA-DQA105, or monitoring of drug levels. An invitation to participate was emailed to a representative number of 244 IBD treating physicians all over the country. The results of the literature review and survey were discussed in a nominal group meeting, and a set of statements were proposed for a Delphi process. The participants of the survey were also invited to the Delphi. Agreement was defined if at least 70% of the participants voted ≥7 (from 1, totally disagree to 10, totally agree). For each statement, the level of evidence and grade of recommendation was established based on the Oxford Evidence Based Medicine categorization.
Results
A total of 14 statements were generated (table 1 depicts the statements and the results of the Delphi). All but two achieved required level of agreement. The statements cover the following areas: 1) use of first-line non-anti-TNF biologic therapy; 2) role of HLA-DQA1*05 in daily practice; 3) attitudes in primary nonresponse and loss of response to anti-TNF therapy due to immunogenicity; 4) ustekinumab or vedolizumab if a change of mechanism of action is considered; 5) anti-TNF drug levels monitoring during induction; 6) combined therapy with an immunomodulator and anti-TNF monotherapy.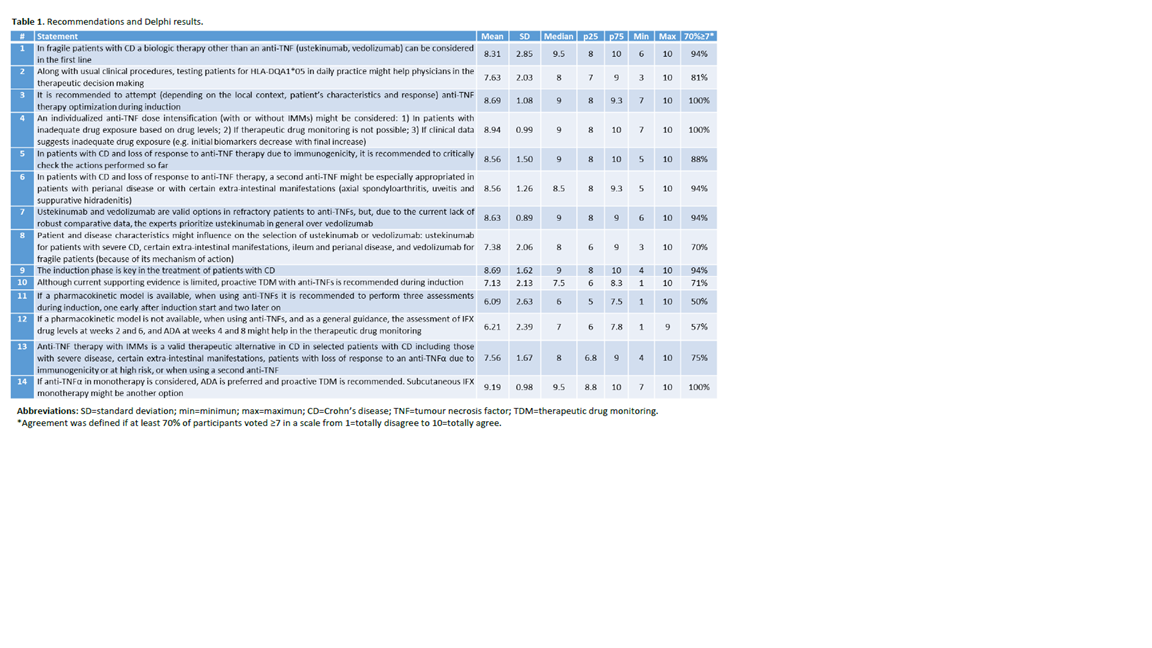
Conclusion
This document sought to pull together the experts’ attitudes when dealing with different clinical scenarios of patients with CD on anti-TNF.


