P663 Post-ustekinumab induction IL12, IL23, and ustekinumab levels are associated with clinical response in a multi-centre prospective cohort study of Crohn’s disease patients: results from the AURORA Study including ANZIBDC Cohort
An, Y.K.(1)*;Lindsay, N.(2);Allan, N.(2);Khoo, E.(1);Fernandes, R.(1);Amiss, A.(2);Pham, H.(2);Wong, K.F.(2);Ooi, S.Y.(3);Thin, L.(4);Lightowler, D.(4);Connor, S.J.(5);Williams, A.J.(5);De Cruz, P.(6);Li Wai Suen, C.(6);Kariyawasam, V.(7);Mitrev, N.(7);Ghaly, S.(8);Andrews, J.M.(9);Christensen, B.(10);Sparrow, M.P.(11);White, L.S.(12);Bryant, R.V.(13);Ding, N.(14);Leong, R.(15);Van Langenberg, D.(16);Seltenreich, H.(17);Subramaniam, K.(18);Radford-Smith, G.(3);Begun, J.(19);
(1)Mater Hospital Brisbane, Department of Gastroenterology, Brisbane, Australia;(2)Mater Research Institute, IBD Research Group, Brisbane, Australia;(3)Royal Brisbane and Women's Hospital, Department of Gastroenterology, Brisbane, Australia;(4)Fiona Stanley Hospital, Department of Gastroenterology, Perth, Australia;(5)Liverpool Hospital, Department of Gastroenterology, Sydney, Australia;(6)Austin Hospital, Department of Gastroenterology, Melbourne, Australia;(7)Blacktown Hospital, Department of Gastroenterology, Sydney, Australia;(8)St Vincent's Hospital Sydney, Department of Gastroenterology, Sydney, Australia;(9)Royal Adelaide Hospital, Department of Gastroenterology, Adelaide, Australia;(10)Royal Melbourne Hospital, Department of Gastroenterology, Melbourne, Australia;(11)Alfred Hospital, Department of Gastroenterology, Melbourne, Australia;(12)Sunshine Coast University Hospital, Department of Gastroenterology, Brisbane, Australia;(13)The Queen Elizabeth Hospital, Department of Gastroenterology, Adelaide, Australia;(14)St Vincent's Hospital Melbourne, Department of Gastroenterology, Melbourne, Australia;(15)Concord Hospital, Department of Gastroenterology, Sydney, Australia;(16)Eastern Health, Department of Gastroenterology, Melbourne, Australia;(17)Costal Digestive Health, Clinical Research Unit, Brisbane, Australia;(18)Canberra Hospital, Department of Gastroenterology, Canberra, Australia;(19)Mater Hospital Brisbane, Department of Gastroenterolgy, Brisbane, Australia; Australia New Zealand IBD Consortium (ANZIBDC)
Background
Ustekinumab (UST) is a monoclonal antibody targeting IL-12 and IL-23 through their shared p40 subunit. This study aimed to determine the clinical outcomes after UST treatment in Crohn’s disease (CD) patients in a real-world setting, and to investigate the association of clinical outcomes with IL-12, IL-23 and UST levels.
Methods
A multi-centre prospective observational cohort study of UST for moderate to severe CD was conducted. Patients were recruited from 19 Australian centres between Sep 2019 and Apr 2022. Clinical assessments were performed at baseline study visit (SV) 1 and post-induction (SV2). Patients were assessed every 6 months during maintenance therapy to 18 months (SV4). Clinical response and remission rates were determined using PRO2 definitions (STRIDE II guidelines). Logistic regression analyses were performed to identify predictors of clinical response and remission. UST levels were measured post induction and interleukin levels including IL12p40 and IL-23p19 were measured at SV1 and SV2 using ProQuantum ELISA assays.
Results
A total of 198 patients were recruited: median age 40.7 years, 54.3% male, median duration of disease 90.5 months, 12.8% active smokers, and 49.7% on concomitant immunomodulatory therapy. The majority (58.4%) were biologic-naïve and 82 patients were previously exposed to biologics (51.9%; IFX n=42, ADA n=50, VDZ n=9). Clinical response was achieved in 137 patients (75.7%), and remission in 84 (46.4%) with higher rates of response (85.2% vs 61.6%, p=0.001) and remission (54.6% vs 34.2%, p=0.006) in biologic naïve patients compared to biologic-exposed.
For the 114 patients with 18 months (SV4) of follow-up, durable clinical response was maintained in 66.8% and remission in 50.0%. Dose escalation (90mg 4 weekly) was administered to 13 patients (6.6%) during induction, 48 (42.1%) in maintenance, and 15 patients (13.2%) received IV re-induction during maintenance.
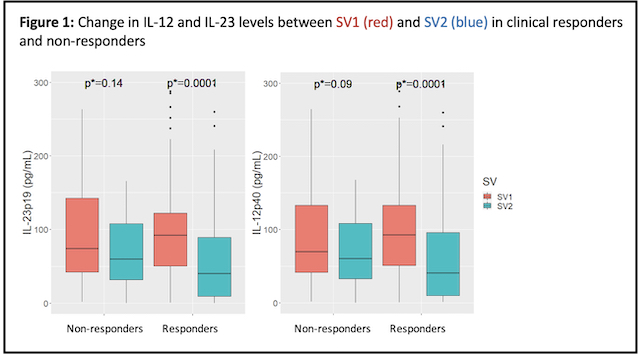
There was a significant reduction in IL-12 and IL-23 levels from SV1 to SV2 in responders (p=0.0001), but no significant reduction in non-responders (Figure 1). Clinical response at SV4 (n=101) was associated with higher post-induction UST levels (p=0.03) (Table 1). The combination of IL-12. IL-23 and UST level at SV2 predicted long-term response at SV4 (Sn 73%, Sp 71%, AUROC 0.765), but not at SV2 (Figure 2).
Conclusion
This large real-world study confirms that UST is most effective in bio-naïve CD patients with significantly higher response and remission rates than bio-experienced patients. The combination of post-induction IL-12, IL-23, and UST levels was associated with response at 18 months and could represent a novel predictor of long-term clinical outcomes.