P680 A real-life experience of Crohn’s Disease Exclusion Diet use at disease onset and as an add-on therapy in pediatric Crohn’s disease
Scarallo, L.(1)*;Fioretti, L.(1);Pochesci, S.(1);Pierattini, V.(2);Banci, E.(2);De Blasi, A.(1);Di Paola, M.(1);Lionetti, P.(1,3);
(1)Meyer Children Hospital, Gastroenterology and Nutrition Unit, Florence, Italy;(2)Meyer Children Hospital, Dietetics Unit, Florence, Italy;(3)University of Florence, Department of NEUROFARBA, Florence, Italy;
Background
We aimed at appraising real-life efficacy of Crohn’s Disease Exclusion Diet (CDED) coupled with partial enteral nutrition (PEN) in inducing clinical and biochemical remission at disease onset and in patients with loss of response to biologics in a tertiary-level center. We also aimed at identifying early responders to this dietary regimen.
Methods
We gathered clinical, anthropometric and laboratory data of patients aged less then 18 years of age with a diagnosis of CD, who were consecutively treated with CDED coupled with PEN as main treatment for the induction of remission or in the setting of loss of response to other therapies. We collected data of patients who received CDED plus PEN from April 2019 to June 2022 at diagnosis, at the beginning of the dietary treatment, and at the phase 1. Patients who interrupted diet during follow-up were considered as treatment failures on an intent to treat basis. We compared groups using chi-square, Fisher’s exact test, Mann-Whitney U or related-samples Wilcoxon signed rank tests or McNemar’s test as appropriate.
Results
47 patients were retrospectively identified. Table 1. summarizes clinical and biochemical characteristics at diagnosis and at CDED with PEN initiation. 24 (51.1%) patients started CDED as induction therapy at disease onset, whereas 23 (48.9%) of them received CDED with PEN as add-on therapy. 32/47 (68%) patients achieved clinical remission (wPCDAI < 12.5) at the end of phase 1, 16/24 patients (66.7%) who started CDED as induction therapy and 16/23 (69.5%) of those who started CDED as add-on, with no statistically significant difference among the two groups (p=1.00). Laboratory parameters significantly improved in both groups (Figure 1.). There were no statistically significant differences in clinical remission rates between patients with mild-to-moderate and severe disease at the end of phase 1 (25/35 vs 7/12, p=0.481). At disease onset, 14 patients added a concomitant treatment (11 patients added anti-TNF alpha, 3 patients added IMM and one patient added anti-TNF alpha + IMM) after a median time of 4 weeks (IQR: 3-5 weeks). 28 patients had clinical data gathered at week 3. Patients who achieved clinical response at week 3 (a change in wPCDAI > 12.5) were more likely to be in clinical remission at the end of phase 1 (17/20 vs 1/8, p<0.01), both in patients who started CDED at disease onset and in the add-on setting.
Conclusion
CDED with PEN confirmed its efficacy in a real-life setting, both as induction regimen and as add-on therapy, also in patients with clinically severe disease. Early clinical response predicts clinical remission at the end of phase 1, possibly allowing identification of dietary responsive disease.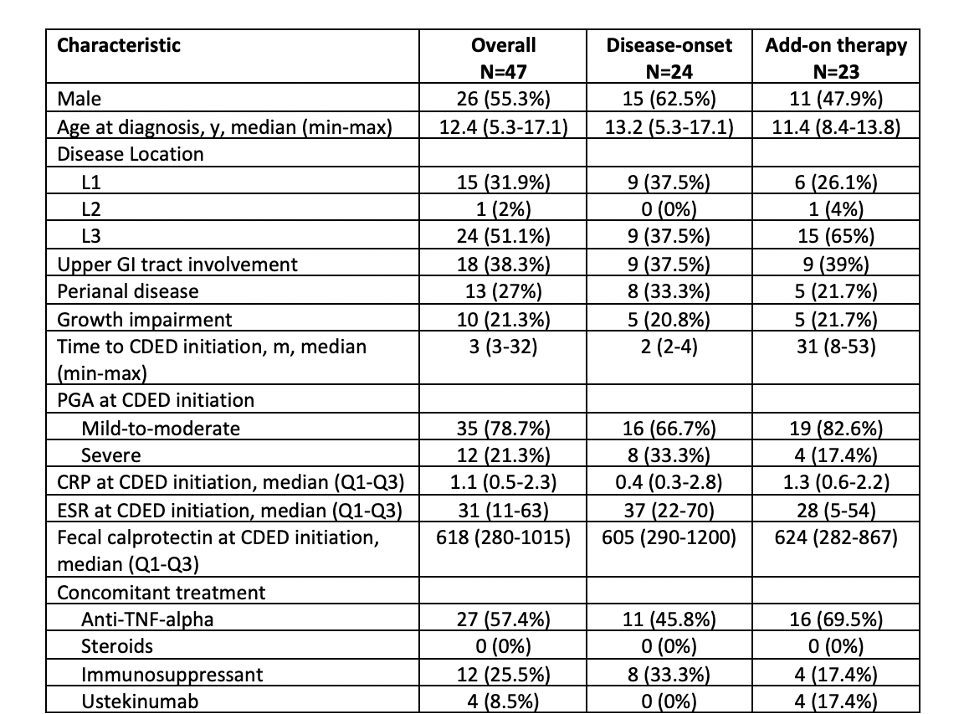
Table 1.
Figure 1.


