P687 Durability of recaptured response to ozanimod during the True North open-label extension
Dignass, A.(1)*;Regueiro, M.(2);Colombel, J.F.(3);Jain, A.(4);Canavan, J.B.(4);Wu, H.(4);Lawlor, G.(4);Osterman, M.T.(4);Vermeire, S.(5);Rubin, D.T.(6);
(1)Agaplesion Markus Hospital- Goethe University, Medicine, Frankfurt, Germany;(2)Cleveland Clinic- Cleveland, Digestive Disease and Surgery Institute, Cleveland, United States;(3)Icahn School of Medicine at Mount Sinai, Gastroenterology, New York, United States;(4)Bristol Myers Squibb, Clinical Research, Princeton, United States;(5)University of Leuven, Chronic Diseases and Metabolism, Leuven, Belgium;(6)University of Chicago, Gastroenterology, Chicago, United States;
Background
Ozanimod is approved in the EU, US, and other countries for treatment of moderately to severely active ulcerative colitis (UC) in adults. In the phase 3 True North (TN) study, ozanimod 0.92 mg once daily demonstrated efficacy and safety for up to 52 weeks in patients (pts) with moderately to severely active UC. This open-label extension (OLE) analysis evaluated the long-term durability of ozanimod based on symptomatic and clinical endpoints in pts who experienced disease relapse while on placebo during the TN maintenance period (MP) and then recaptured response to ozanimod during the OLE.
Methods
Responders to ozanimod 10-week induction therapy were rerandomised 1:1 to ozanimod (n=230) or placebo (n=227); this OLE interim analysis (data cutoff: January 10, 2022, at which point the OLE Week 94 disposition was available for pts) included all pts who relapsed on placebo during the MP and reinitiated ozanimod in the OLE. Endpoints included symptomatic response and remission, clinical response and clinical remission (which include endoscopy subscores), and corticosteroid (CS)-free remission (clinical remission while off CS for ≥12 weeks) evaluated using observed case (OC) and nonresponder imputation (NRI) analyses, and mean total Mayo score over time.
Results
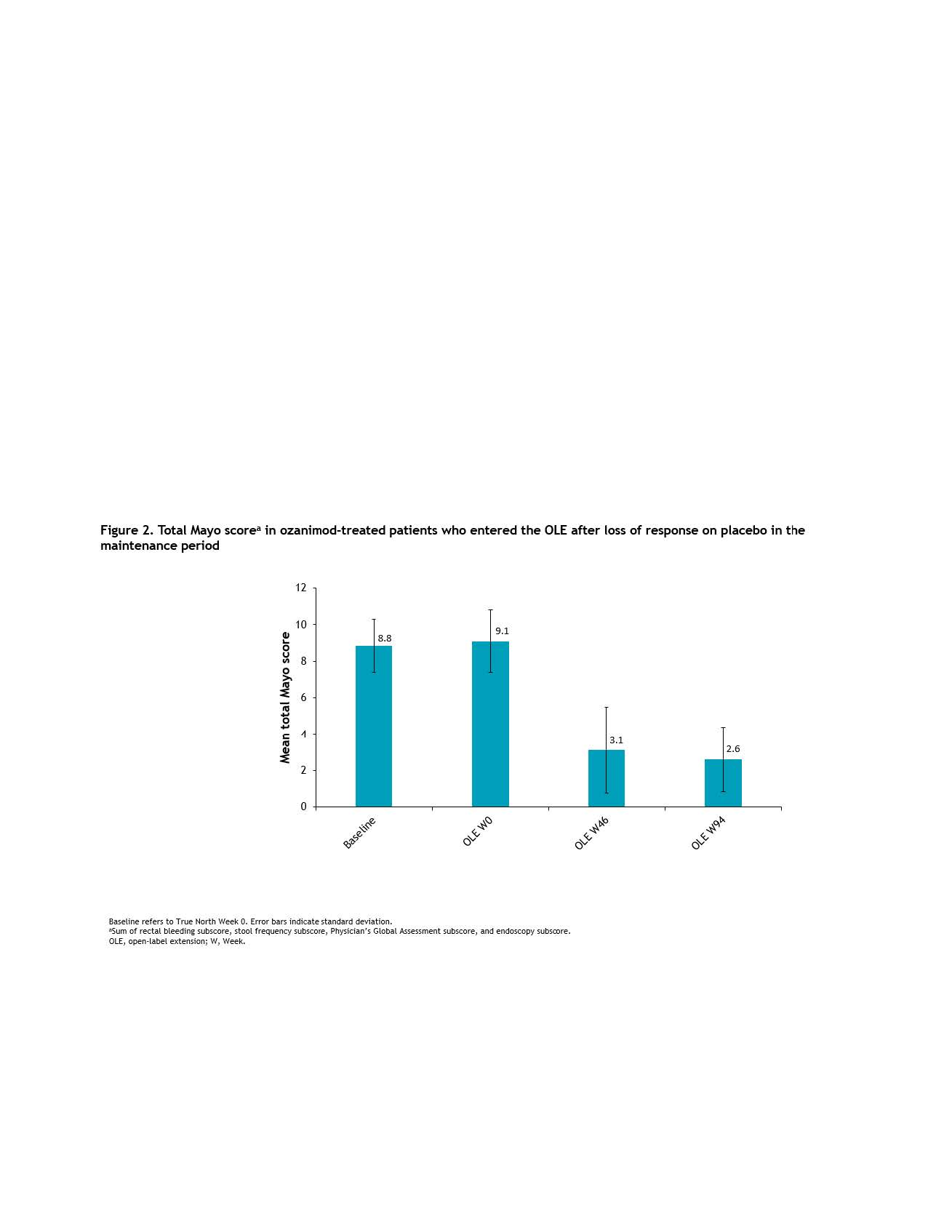
A total of 77/227 pts (34%) rerandomised to placebo during MP experienced a relapse after a median of 12.7 weeks and all entered the OLE. Symptomatic response was achieved by 46/73 (63%) pts 5 weeks after restarting ozanimod in the OLE (NRI analysis: 60% at OLE Week 5) and in 32/33 (97%) by OLE Week 94 in the OC analysis. Symptomatic remission was also achieved by OLE Week 5 by 24/73 (33%) pts. Sustained efficacy of ozanimod reinitiation was also demonstrated comparing OLE Weeks 46 and 94 across clinical endpoints by OC analysis (clinical response, 76% and 86%; clinical remission, 44% and 59%; CS-free remission, 37% and 52%) and NRI analysis (clinical response, 42% and 31%; clinical remission, 25% and 22%; CS-free remission, 21% and 20%) (Figure 1). In addition, 56.3%, 52.6%, and 50% of pts who experienced clinical response, clinical remission, and CS-free remission, respectively, at OLE Week 46 continued to sustain these endpoints at OLE Week 94. Reductions in mean total Mayo score at OLE Week 46 were also sustained through OLE Week 94 (Figure 2).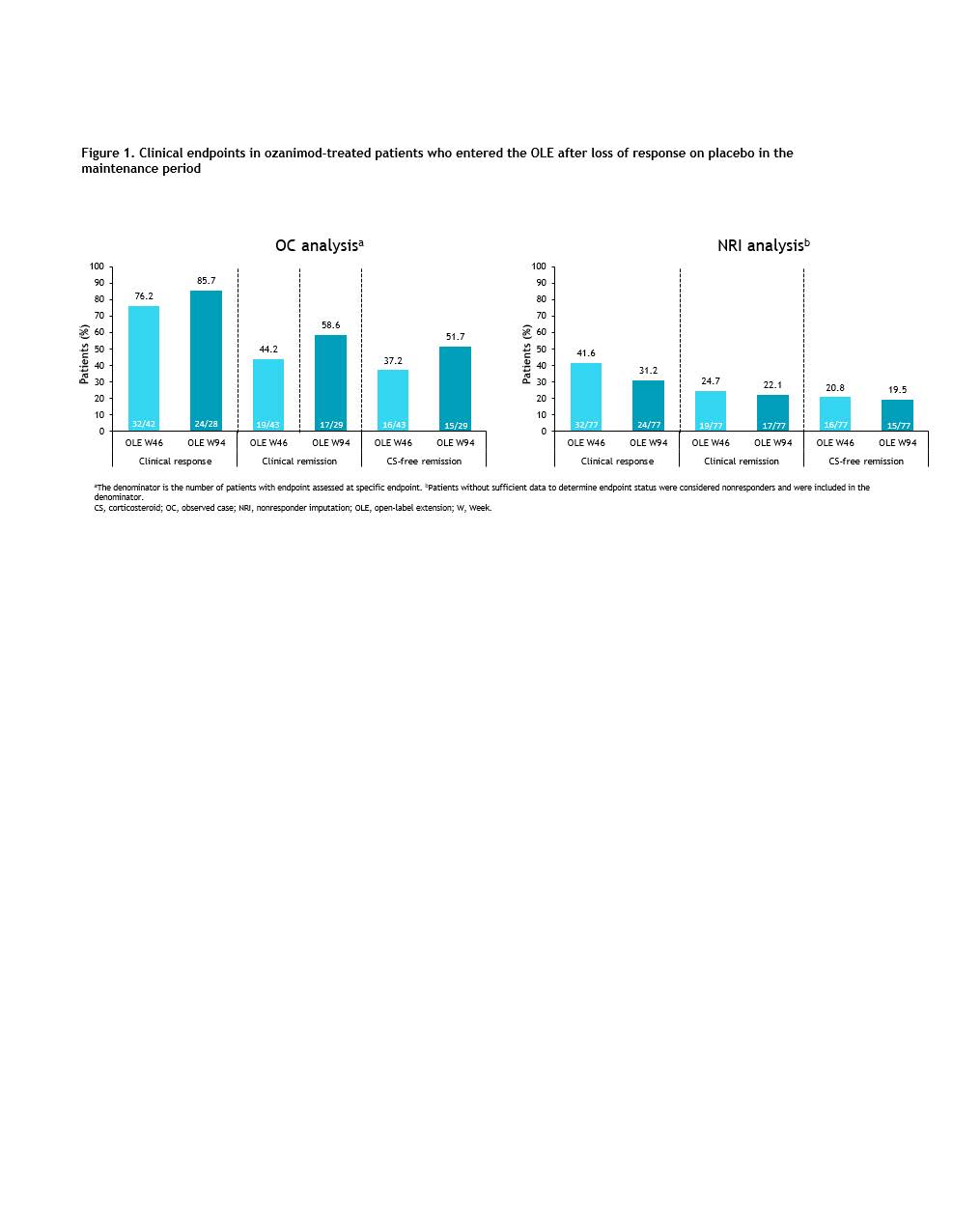
Conclusion
Response to ozanimod was regained after temporary therapy discontinuation for most pts, and this response was durable for up to 2 years as demonstrated by both symptomatic and clinical endpoints in this interim analysis from the TN OLE study. These results suggest that pts who experienced disease relapse after temporarily holding ozanimod in clinical practice may benefit from resumption of ozanimod.


