P687 Early and sustained improvement in stool frequency and rectal bleeding following 52 weeks of mirikizumab treatment
G.R. D’Haens1, T. Hibi2, M. Ferrante3, B.R. Bhandari4, E. Berliba5, J.L. Tuttle6, T. Lissoos7, K. Krueger7, N. Morris7, V. Arora7, A.N. Naegeli7, B.G. Feagan8
1Amsterdam University Medical Centers, Inflammatory Bowel Disease Centre, Amsterdam, The Netherlands, 2Kitasato Institute Hospital, Center for Advanced IBD Research and Treatment, Tokyo, Japan, 3Universitair Ziekenhuizen Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium, 4Delta Research Partners, n/a, Bastrop, LA, USA, 5Nicolae Testemitanu State University of Medicine, Arsenia Exploratory Medicine, Chisinau, Moldova Republic of, 6Eli Lilly and Company, Lilly Biotechnology Center, San Diego, CA, USA, 7Eli Lilly and Company, Lilly Biomedicines, Indianapolis, IN, USA, 8Western University, Robarts Clinical Trials Inc., London, ON, Canada
Background
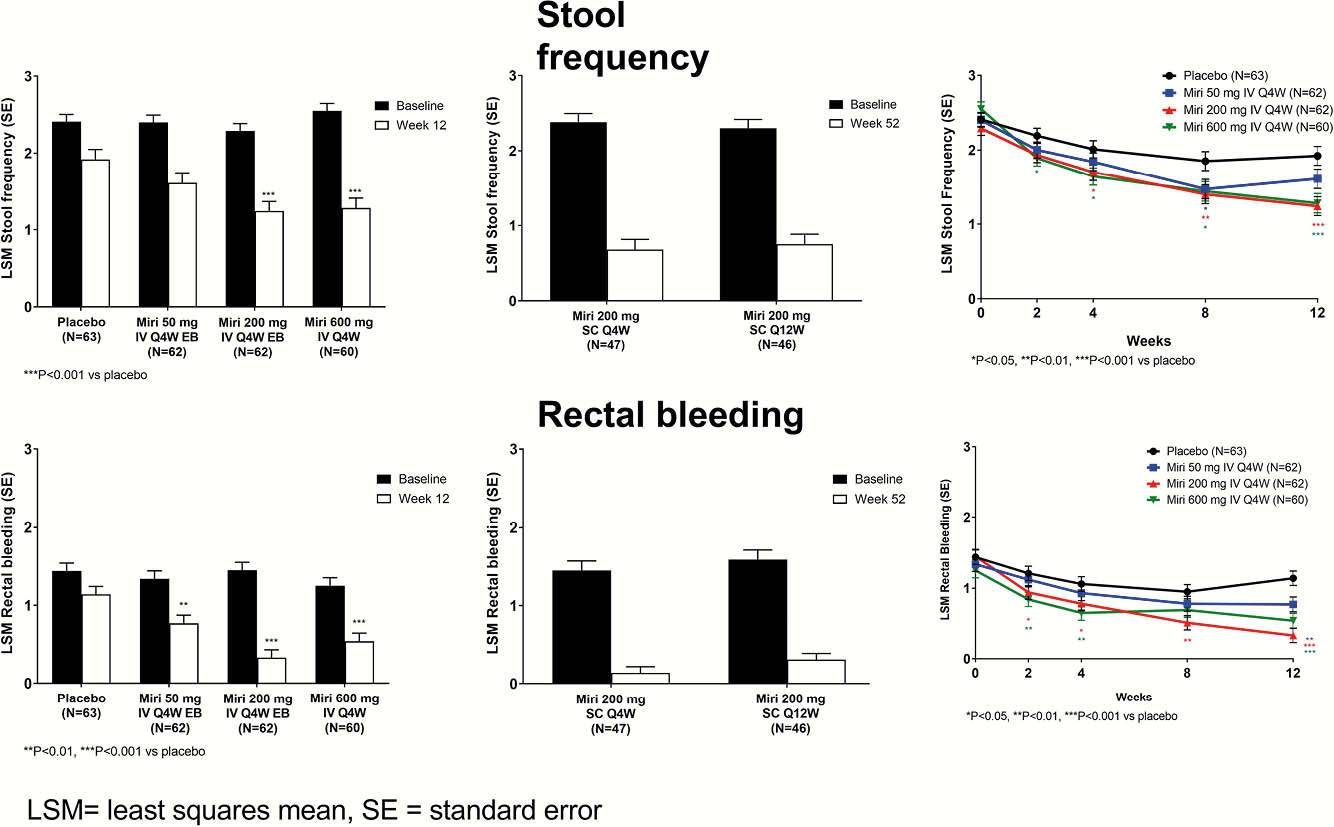
Interleukin (IL)-23 is a key cytokine in inflammatory bowel disease pathogenesis. Mirikizumab (miri), a p19-directed IL-23 antibody, demonstrated efficacy and was well-tolerated in patients with moderate-to-severely active ulcerative colitis (UC) during 52 weeks of a Phase 2 randomised clinical trial (AMAC, NCT02589665). The effects of miri on stool frequency (SF) and rectal bleeding (RB) through Week 52 are reported.
Methods
Patients (Mayo score 6–12 with endoscopic subscore [ES] ≥2) were randomised 1:1:1:1 to receive intravenous (IV) placebo (
Results
Significant improvement in SF was observed as early as Week 2 in the miri 600 mg Q4W group and as early as Week 4 in the miri 200 mg Q4W group (Figure). Similarly, significant improvement in RB was observed as early as Week 2 in both the miri 200 mg Q4W and 600 mg Q4W groups. Patients who received miri 200 mg Q4W or 600 mg Q4W had a significantly greater reduction in both SF and RB compared with placebo at Week 12 (all
Conclusion
Significant improvements in SF and RB occurred as early as Week 2 of induction treatment and continued to improve during maintenance treatment with mirikizumab. These data demonstrate the early efficacy and longer-term effects of an IL-23p19 antibody on these patient-reported outcomes.


