P688 Effect of golimumab induction therapy on histologic disease profile in patients with moderate to severe ulcerative colitis: Results from the randomised, placebo-controlled Phase 3 PURSUIT-SC trial
T. Johansson1, C. Weinstein2, A. MEEHAN3, A. Tzontcheva4, P. Xu4, C. Marano5, G. de Hertogh6
1MSD, Medical Affairs, Kriens, Switzerland, 2Merck and Co. Inc., Clinical Research, Kenilworth, USA, 3Merck and Co. Inc., Global Scientific and Medical Publications, Kenilworth, USA, 4Merck and Co. Inc., Biostatistics, Kenilworth, USA, 5Janssen Research and Development, LLC, Clinical Research, Spring House, USA, 6University Hospitals KU Leuven, Pathology, Leuven, Belgium
Background
Histologic improvement is an emerging goal in ulcerative colitis (UC) management. We previously demonstrated in the 6-week, multicentre, randomised, placebo-controlled, double-blind Program of Ulcerative colitis Research Studies Utilising an Investigational Treatment [PURSUIT-SC] Phase 3 trial that SC induction therapy with the tumour necrosis factor-α inhibitor, golimumab (GLM), led to statistically significant and clinically meaningful improvements in the signs and symptoms of UC compared with placebo, including clinical remission, mucosal healing, and improved health-related QoL, in patients with moderate to severe active UC.1 In the present report, we explored the histologic effects of GLM induction therapy in PURSUIT-SC by examining data from a prespecified histology sub-study.
Methods
The Phase 3 PURSUIT-SC trial evaluated the safety and efficacy of induction therapy with GLM in 774 patients with moderate to severe UC disease activity (defined as Mayo scores of 6–12, including an endoscopic subscore of ≥ 2). Patients were randomised 1:1:1 to receive SC induction treatment with placebo at Weeks 0 and 2, GLM 200 mg at Week 0 followed by 100 mg at Week 2 (GLM 200
Results
Evaluable biopsy data were available in 89 patients (placebo,
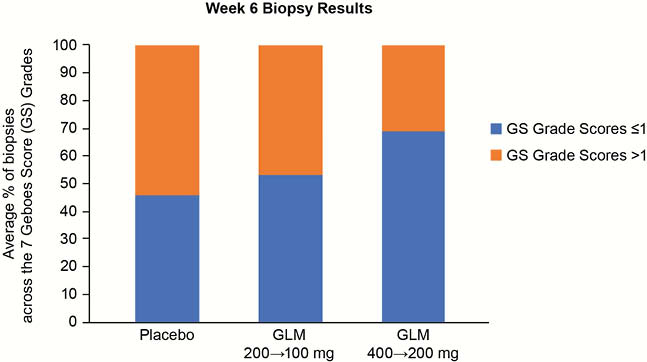
Conclusion
The findings of this exploratory histologic sub-study in the PURSUIT-SC trial suggest that GLM induction therapy led to an improvement in histologic disease activity compared with placebo in patients with moderate-to-severe UC.
Sandborn WJ,


