P693 Italian real-life study evaluating the long-term effectiveness of vedolizumab for the treatment of Inflammatory Bowel Disease- An IG-IBD study
Pugliese, D.(1);Privitera, G.(2)*;Armuzzi, A.(3);
(1)Fondazione Policlinico Agostino Gemelli IRCCS, CEMAD- IBD CENTER- Unità Operativa Complessa di Medicina Interna e Gastroenterologia- Dipartimento di Scienze Mediche e Chirurgiche, Rome, Italy;(2)Università Cattolica del Sacro Cuore, Dipartimento di Medicina e Chirurgia Traslazionale, Roma, Italy;(3)IRCCS Humanitas Research Hospital, IBD Center, Rozzano, Italy; IG-IBD LIVE Studygroup
Background
Real-world studies on vedolizumab (VDZ) are often limited by small sample size and short follow-up and there is still lack of knowledge regarding predictors of response. Our study aims to evaluate the long-term effectiveness and safety of VDZ in a large cohort, and to identify clinical features for precision medicine.
Methods
The Long-term Italian VDZ Effectiveness (LIVE) study included CD and UC patients started on VDZ from 04/2016 to 06/2017 at 47 Italian centers and prospectively followed-up until 06/2019. The primary endpoint was steroid-free clinical remission (SFCR) at 12 months (mo), defined as clinical remission + no steroid therapy in the previous 3 mo. Secondary endpoints included treatment persistence and safety. An eXplainable Artificial Intelligence (XAI) model (XGBoost) was used to predict 12 mo-SFCR. To assess the model performances, F1-score was adopted.
Results
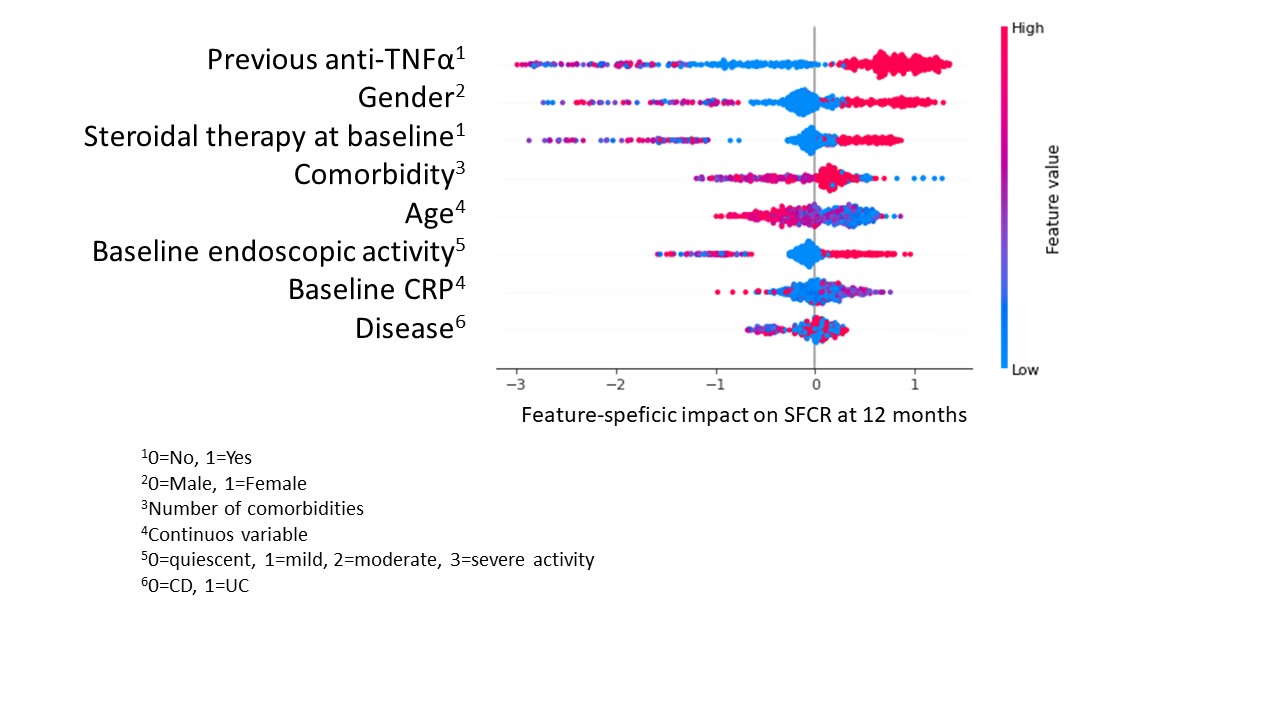
We enrolled 1111 patients (564 CD, 547 UC). At baseline, mean age and disease duration were 47.7(±16) and 11.8(±9) years; 256 (23%) patients were bionaïve. SFCR was reported in 193 (34.3%) and 178 (31.6%) CD patients, and in 205 (41.9%) and 215 (42.6%) UC patients, at 12 and 24 mo (p=ns for all). Cumulative treatment persistence at 12 and 24 mo was 73.3% and 67.3%, respectively. A significantly longer persistence was observed among bionaïve compared to bioexperienced CD patients (p=0.04), but not in UC. At least 1 adverse event (AE) was experienced by 230 patients (20.8%), causing VDZ withdrawal in 58 (5.2%) of them; the most common AEs were infections. The trained machine learning (ML) algorithm was used to predict 12 mo-SFCR. To minimize the bias introduced by the imbalanced dataset, a data augmentation technique (Synthetic Minority Over-sampling Technique, SMOTE) was introduced. The predictive model’s accuracy (F1 score) was 0.85. To explore the relations among the variables for the predicted outcome, Shapley Additive exPlanations (SHAP) was adopted. Figure 1 presents the 8 best features, hierarchically listed, with their average impact on the outcome. Figure 2 shows how the value taken by each feature contributes to predicting 12 mo-SFCR (dots close to the 0 line predict SFCR). The most important variables. correlating with 12 mo-SFCR were No exposure to anti-TNF, male sex and no baseline steroid therapy.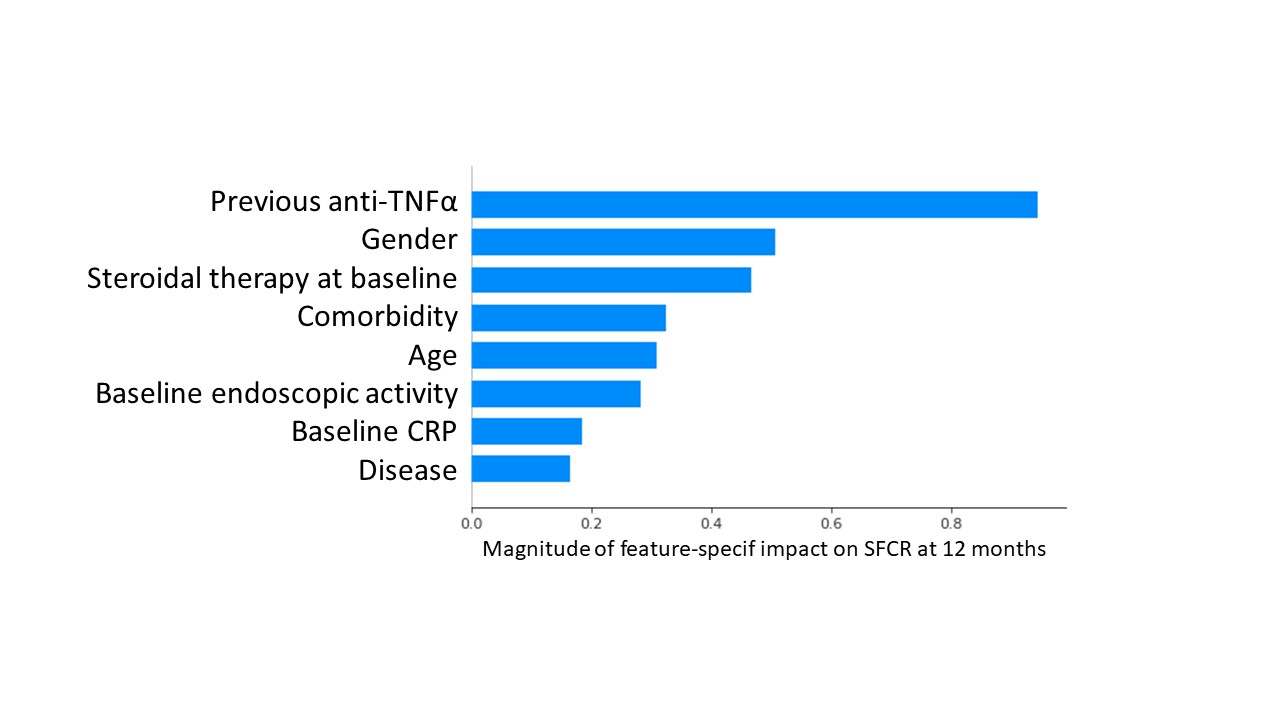
Conclusion
In this preliminary analysis, about 1/3 of VDZ-treated patietns achieved 12 mo-SFCR. With ML, we identified the most important predictors of SFCR and estimated their impact on it. XAI is a promising tool in precision medicine, as it helps reconciling sophisticated models with the biological explainability of data.


