P696 Design and rationale for the multicentre, randomised, controlled VERDICT trial to determine the optimal treatment target in patients with ulcerative colitis
Jairath, V.(1,2,3)*;Zou, G.(2,3,4);Radulescu, G.(3);Sigler, J.(3);Mcfarlane, S.C.(3);Adsul, S.(5);Freire, M.(5);Peyrin-Biroulet, L.(6);Colombel, J.F.(7);Moran, G.W.(8,9);Sebastian, S.(10,11);Travis, S.(12);Vermeire, S.(13);Sandborn, W.J.(14);D’Haens, G.R.(15); Feagan, B.G.(1,2,3);
(1)University of Western Ontario, Department of Medicine- Division of Gastroenterology, London, Canada;(2)University of Western Ontario, Department of Epidemiology and Biostatistics, London, Canada;(3)Alimentiv Inc., Medical Research and Development, London, Canada;(4)University of Western Ontario, Robarts Research Institute- Schulich School of Medicine and Dentistry, London, Canada;(5)Takeda Pharmaceuticals, Medical, Cambridge, United States;(6)Inserm NGERE U1256- University Hospital of Nancy- University of Lorraine, Department of Gastroenterology, Vandoeuvre-lès-Nancy, France;(7)Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, New York, United States;(8)University of Nottingham, Nottingham Digestive Diseases Biomedical Research Centre, Nottingham, United Kingdom;(9)Nottingham University Hospitals NHS Trust, NIHR Nottingham Biomedical Research Centre, Nottingham, United Kingdom;(10)Hull Royal Infirmary, Hull and East Yorkshire NHS Trust & Hull and York Medical School, Hull, United Kingdom;(11)Hull York Medical School, School of Health & Life Sciences, Hull, United Kingdom;(12)University of Oxford, Kennedy Institute and Biomedical Research Centre, Oxford, United Kingdom;(13)University Hospitals Leuven- KU Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(14)University of California San Diego, Division of Gastroenterology, La Jolla, United States;(15)Amsterdam University Medical Centers, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands; on behalf of the VERDICT Study Group
Background
Symptoms, endoscopy, histology, and biomarkers are used to evaluate disease activity and response to therapy in ulcerative colitis (UC). Histologic disease activity may persist in ~25% of patients with normal-appearing endoscopic mucosa, and observational studies demonstrate an association between the achievement of histologic remission (vs endoscopic remission alone) and a lower risk of complications. These findings suggest that histologic remission may be a distinct treatment target. The aim of the VERDICT trial is to determine the optimal treatment target in UC to inform clinical practice and future drug development.
Methods
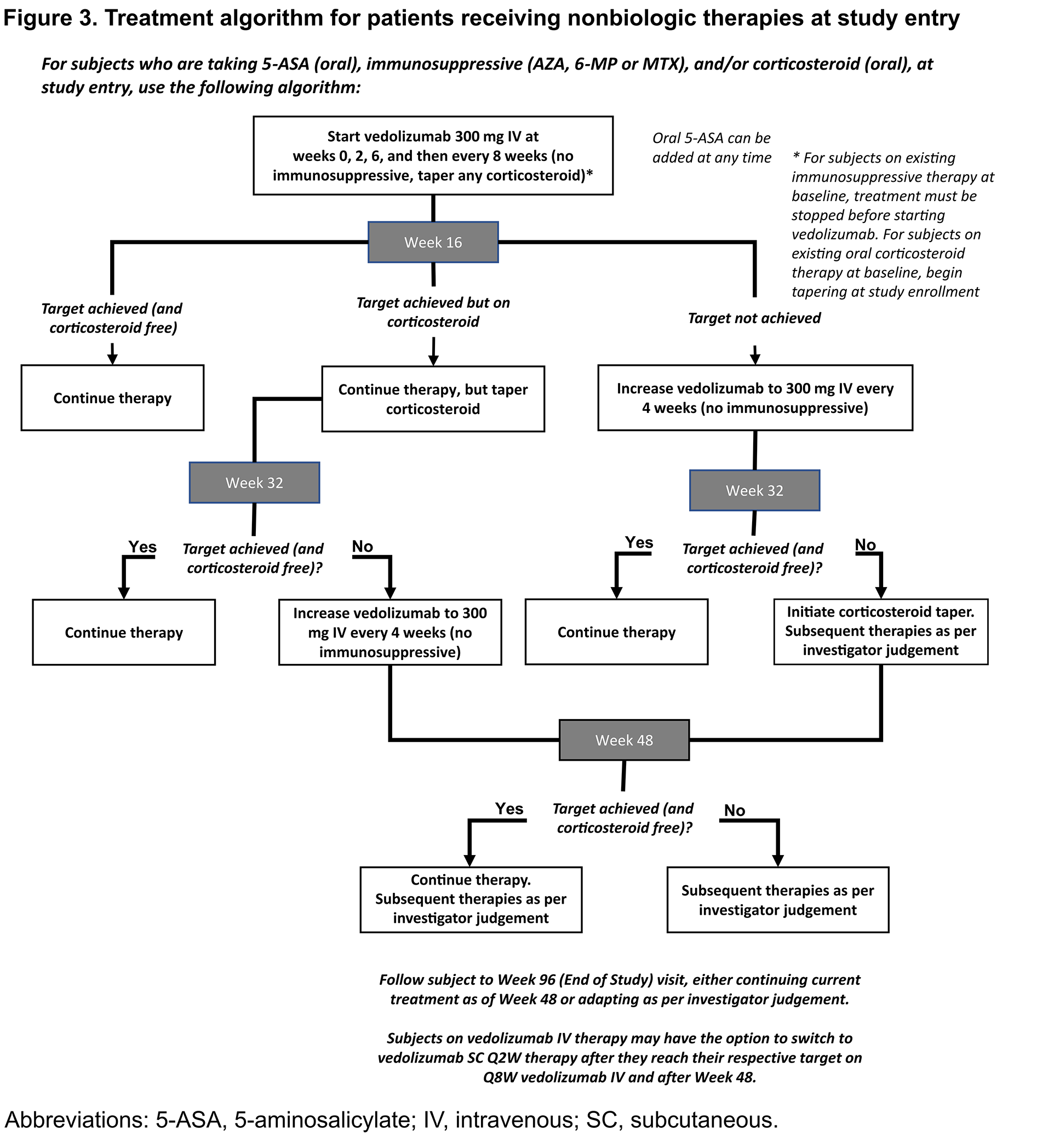
The primary objective of the multicentre, randomised, controlled VERDICT trial (Figure 1) is to determine whether a treatment target of corticosteroid (CS)-free symptomatic + endoscopic + histologic remission is superior to CS-free symptomatic remission alone in moderately to severely active UC (Mayo rectal bleeding subscore [RBS] ≥1; Mayo endoscopic score [MES] ≥2). Patients are randomised to 3 treatment targets (2:3:5 ratio): CS-free symptomatic remission (Mayo RBS = 0) (Group 1); CS-free endoscopic remission (MES ≤1) + symptomatic remission (Group 2); or CS-free histologic remission (Geboes score <2B.0) + endoscopic remission + symptomatic remission (Group 3). Therapy is administered according to a treatment algorithm that is dependent on each patient’s existing UC treatment regimen at study entry (Figures 2-4). Early introduction of vedolizumab and dose escalation to a maximum of 300 mg every 4 weeks until the assigned treatment target is reached are central to each algorithm. There are 3 opportunities, 16 weeks apart (weeks 16, 32, or 48), to achieve the treatment target. Urine, stool, mucosa, and serum samples are collected at these follow-up time points and at baseline for subsequent biomarker and prediction model development.


Results
The primary efficacy outcome is the time from treatment target achievement to a UC-related complication, defined as a UC-related hospitalisation, colectomy, need for rescue therapy, or treatment-related or other complication. This composite outcome will be compared among treatment target Groups 1 and 3 using the Cox proportional hazard model analysis. The trial was initiated in September 2020, and 660 patients are planned for enrolment. Of these, ~50% are currently enrolled at 55 sites across 10 North American and European countries. Prespecified interim analyses will be conducted when the first 50 patients in each group reach weeks 16, 32, and 48.
Conclusion
The VERDICT trial in active UC is designed to determine the optimal treatment target to inform clinical practice, drug trials, and future evidence-based recommendations.


