P697 De-escalating therapy in inflammatory bowel disease: Results from an observational study in clinical practice.
Arenas, A.(1,2,3)*;Moreta, M.J.(4);Ordás, I.(1,4);Fernández-Clotet, A.(1,4);Caballol, B.(1,4);Gallego, M.(1,4);Vara, A.(1,4);Barastegui, R.(1,4);Giner, A.(1,4);Prieto, C.(1,4);Masamunt, M.C.(1,4);Ricart, E.(1,4);
(1)Hospital Clínic, Inflammatory Bowel Disease Unit, Barcelona, Spain;(2)Complejo Asistencial Dr. Sótero del Río, Unidad de Gastroenterología, Santiago, Chile;(3)Facultad de Medicina Clínica Alemana-Universidad del Desarrollo, Gastroenterología, Santiago, Chile;(4)Hospital Clínic, Gastroenterology, Barcelona, Spain;
Background
Combination therapy with an immunomodulator (IMM) and an anti-TNF agent (specifically infliximab) is recommended in Crohn’s disease (CD) and ulcerative colitis (UC) patients to improve efficacy and reduce anti-TNF immunogenicity. Combo therapy increases the risk of adverse events and therefore attempts are made to de-escalate therapy in clinical practice. There are scarce data about relapse rates after discontinuation of either IMM or anti-TNF among patients with long-term remission under combination therapy. The aims of this study were to assess the risk of relapse in a cohort of UC and CD patients with long-standing remission after discontinuation of IMM or anti-TNF and to identify predictive factors for relapse.
Methods
This is a retrospective unicentric study that included patients with UC or CD on combination therapy and sustained clinical remission at least for 6 months. IMM or anti-TNF were stopped upon treating-physician decision and data were collected until relapse or last follow-up. Relapse was defined as the onset of clinical, biological, endoscopic or radiological activity, leading to therapeutic intervention. The survival without relapse is represented by Kaplan–Meier curve and analyzed by log rank test. Hazard ratios (HR) for relapse were estimated by Cox proportional hazard regression analyses and are reported with 95% confidence intervals (CI).
Results
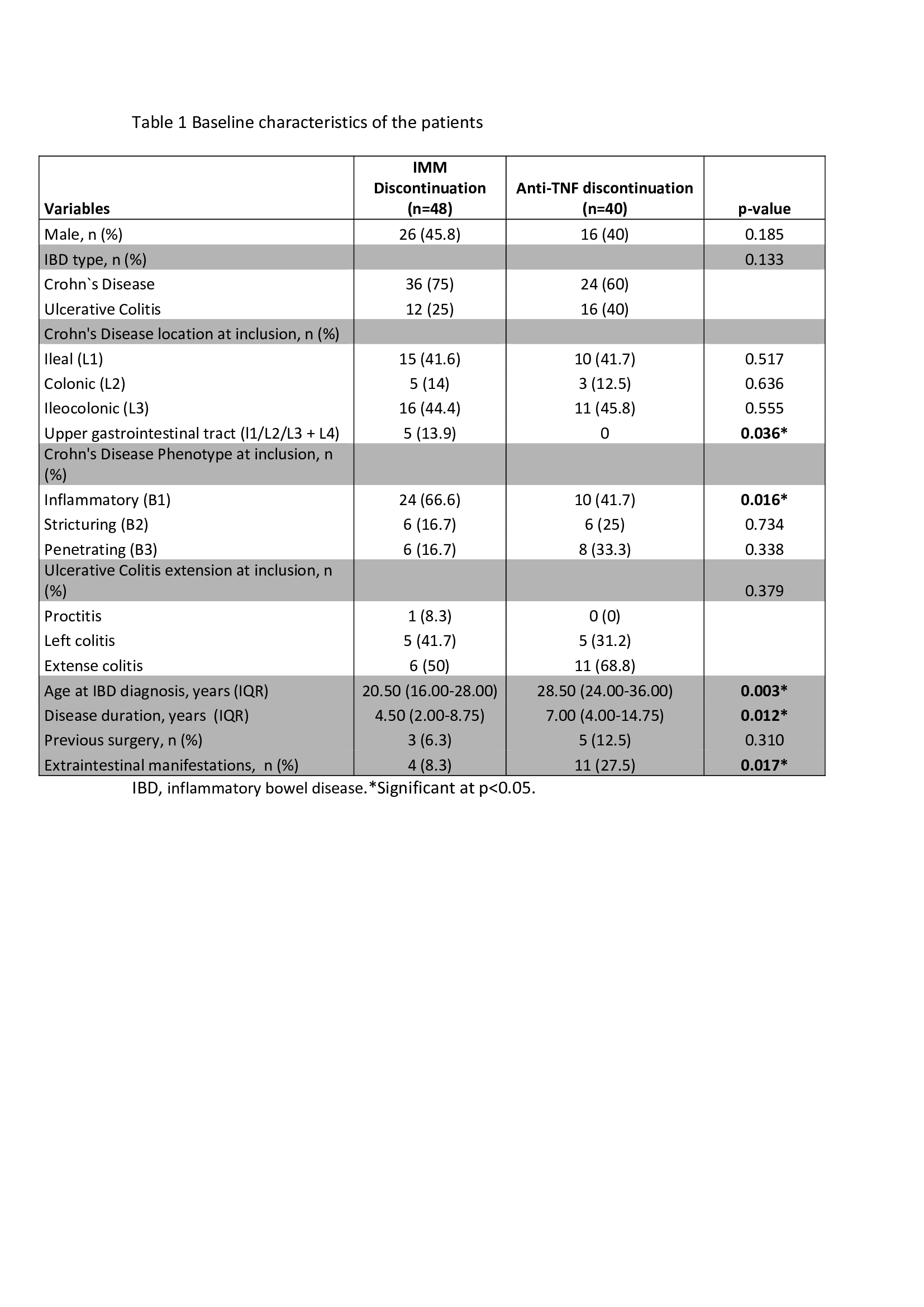
Eighty-eight patients (60 CD (68.2%), 28 UC (31.8%) were included. Demographic data and clinical characteristics are shown in tables 1 and 2. Forty-eight patients (54.5%) discontinued IMM and 40 (45.5%) anti-TNF. Median time of follow-up was 33 months (IQR 18.00-55.75). During follow-up, relapse rate was 16.7% in the IMM discontinuation group and 52.5% in the anti-TNF discontinuation group (p<0.001) (Figure 1). Median time without relapse after discontinuation of IMM and anti-TNF was 38.50 (IQR 19.75-58.5) and 24.50 months (IQR 10.25-42.75), respectively (p=0.036). In the multivariate analysis, only discontinuation of anti-TNF was a predictive factor for relapse (HR=4.99; 95% CI=1.11–14.07) (Table 3). Among patients who relapsed after anti-TNF discontinuation, clinical response after reintroduction of the drug was achieved in 78.6% (11/14) without safety issues.
Conclusion