P697 Efficacy and safety of ustekinumab in Crohn’s disease: A real-world study from Australia
P. Kakkadasam Ramaswamy, H. Moattar, E. Sawyer, J. Edwards, D. Shukla
Department of Gastroenterology, Gold Coast University Hospital, Southport, Australia
Background
Ustekinumab (UST), a human anti-IL12/23p40 monoclonal antibody, was approved in Australia for the treatment of adults with moderate to severe Crohn’s disease (CD) in 2017. The aim of this retrospective single centre study was to study the efficacy and safety of UST in CD in a real world cohort.
Methods
Patients with CD who began UST therapy between June 2017 and July 2019 were included. UST induction was given as an infusion (6 mg/kg) at week 0 followed by 90 mg subcutaneous injection (SC) at week 8 and 90 mg SC every 8 weeks as maintenance. Primary endpoint (PE) was steroid free clinical remission or steroid free clinical response at week 24. Secondary endpoints (SE) were: endoscopic response or remission, radiological response or remission, biochemical response (CRP < 5 mg/L or Calprotectin <150 μg/g), clinical response or remission at week 52.
Results
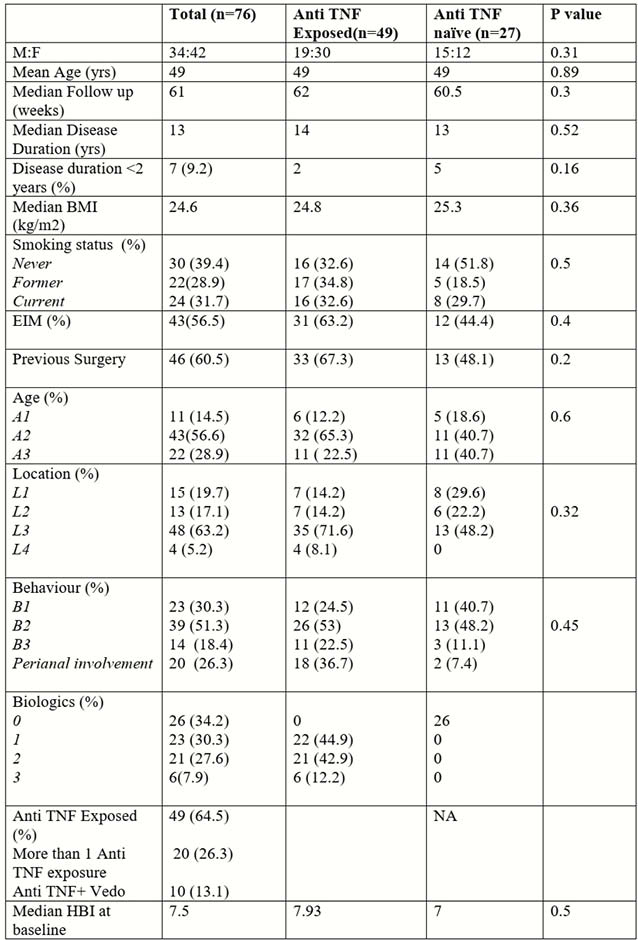
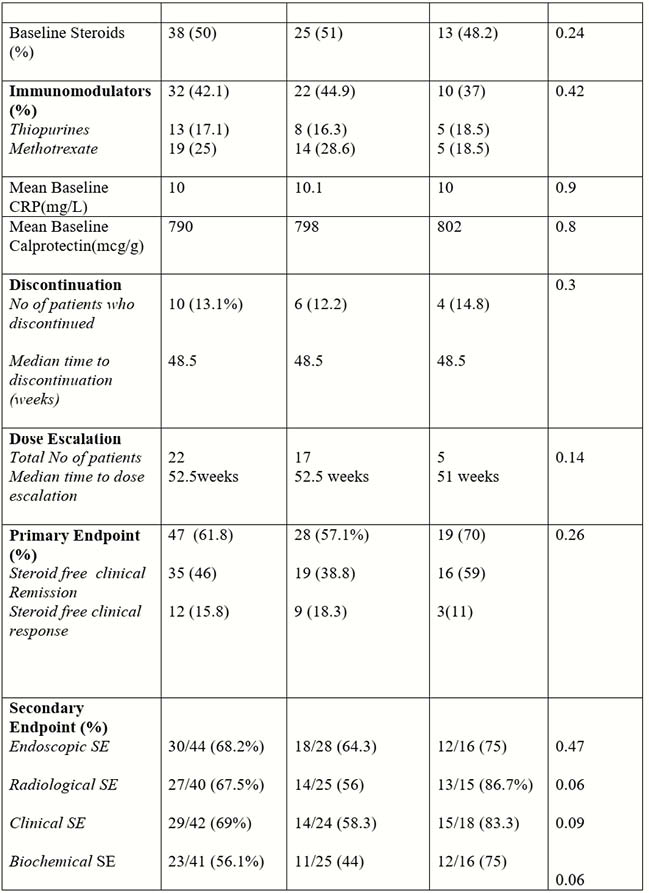
Seventy-six patients with CD were included in the study. 64.5% failed ≥1 anti-TNF and 13.1% failed anti-TNF and vedolizumab; 26(34.2%) patients were biologic-naïve. Median follow-up was 61 weeks. Ten patients (13.1%) discontinued UST, and the median time to discontinuation was 48 weeks; eight patients due to loss of response, one patient due to paradoxical worsening of arthralgia, one patient chose to stop treatment. Six patients underwent surgery whilst on UST. At week 12, 48 (63.1%) of patients achieved steroid free clinical remission or response [19/26 (73%) Anti-TNF naive and 29/49 (59%) anti-TNF exposed]; of these patients, 16/19 (84%) in the anti-TNF naive group and 19/29(65.5%) in the anti-TNF exposed group achieved PE. Forty-seven (61.8%) patients achieved PE. At 52 weeks, 68.2% (30/44), 67.5% (27/40), 56.1% (23/41), 69% (29/42) achieved endoscopic, radiological, biochemical and clinical endpoints respectively. Achieving steroid free clinical response or remission at week 12 was associated with achieving PE (79% vs. 42.8%, OR 3.6,
Conclusion
In a real-world cohort, UST appears efficacious and safe in medium and long term, with modest clinical, biochemical, radiological and endoscopic outcomes. Patients who achieve steroid-free clinical remission or response at 12 weeks are more likely to be in clinical remission or response at 24 and 52 weeks.


