P703 Real world experience of the transition from the intravenous to subcutaneous formulation of vedolizumab in inflammatory bowel disease: A review of current data
Agboton, C.(1)*;Kamble, P.(1);
(1)Takeda, Takeda, Cambridge- MA, United States;
Background
Vedolizumab (VDZ), a gut selective antilymphocyte trafficking anti-α4β7 integrin monoclonal antibody indicated for the treatment of IBD. After a 3 doses induction, maintenance can be achieved with a standard 8-weekly (Q8W) regimen, or–at any time during the maintenance– patients can be transitioned to 108 mg subcutaneous (SC) formulation at a standard 2 weekly (Q2W) regimen.
Methods
This was a literature review to identify real-world studies on the transition from the IV to SC VDZ. The Pubmed and Embase databases were searched up to October 2022 to identify publications on real-world data on the transition from IV to SC VDZ.
Results
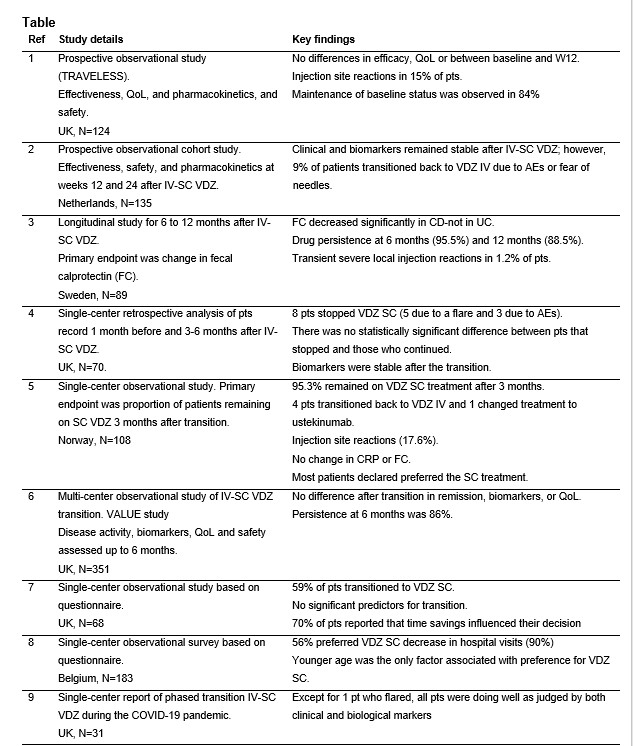
In total, 9 studies were identified with a total of 1,323 IBD patients in the UK (5 studies), Norway, Sweden, Belgium and The Netherlands1-9. Two prospective studies reported no loss of effectiveness based on disease activity scores of VDZ after the IV-SC transition 1,2, along with maintenance of baseline status (status at the time of transition) reported in 84% of patients1. Biomarkers stability, including fecal calprotectin and C-reactive protein, were reported in three studies3-5. Persistence with VDZ treatment following IV-SC transition was 96% at 6 months and 89% at 12 months in one study3, while another study reported persistence of 86% at 6 months6. A preference for the SC formulation by patients was reported 59% and 56% in two studies7,8, with time savings and fewer hospital visits recorded as the main reasons for preference of the SC formulation7. Multivariate analysis of patient characteristics in two studies7,8 revealed no factor associated with preference for VDZ SC other than younger age in one study8. The most frequently reported adverse events with VDZ SC were injections site reactions: 15% and 18% in two studies1,5, with transient severe injection site reactions reported in 1% of patients in one study3.
Conclusion
Real-world data on IBD patients who transitioned from IV to SC VDZ showed no change in effectiveness, with a majority preferring the SC formulation due to time savings and the transition leading to fewer hospital visits. Safety of VDZ SC was generally comparable with that of IV VDZ.
References
1. Ventress E et al J Crohn’s Colitis 2022;16:911-21 2. Volkers A et al Aliment Pharmacol Ther 2022;56:1044-54 3. Bergqvist V et al Aliment Pharmacol & Ther 2022;55:1389-1401 4. Mclean M et al Gut 2022;71:A58-A59 5. Wiken T et al J Crohn’s Colitis 2022;16:i378-79 6. Lim S et al J Crohn’s Colitis 2022;16:i499-i500 7. Alice H et al J Crohn’s Colitis 2022;16:i410 8. Asnong K et al J Crohn’s Colitis 2022;15:S608-9 9. Maw F et al Gut 2021;70:a91