P713 Proactive therapeutic infliximab monitoring during induction phase in inflammatory bowel disease
Gonzalez Vivo, M.(1,2)*;Carballo Martinez, N.(2,3);Padulles Zamora, N.(4);Colominas Gonzalez, E.(2,3);Murciano Gonzalo, F.(1);Abril Rodriguez, L.(1);Marquez-Mosquera, L.(1,2);
(1)Hospital del Mar, Gastroenterology department, Barcelona, Spain;(2)IMIM, Hospital del Mar Medical Research Institute, Barcelona, Spain;(3)Hospital del Mar, Pharmacy department, Barcelona, Spain;(4)Hospital Universitari de Bellvitge, Pharmacy department, L'Hospitalet de Llobregat, Spain;
Background
Therapeutic drug monitoring (TDM) of infliximab (IFX) in patients with Crohn’s disease (CD) and ulcerative colitis (UC) is increasingly used in clinical routine, although proactive TDM is still controversial. Nevertheless, disease activity and drug clearance are higher at the induction phase leading to an increased risk of inadequate drug exposure and treatment failure.
The aim of the study was to evaluate the efficacy of the implementation of an early proactive TDM strategy, comparing clinical remission (CR) and biochemical remission (BR) at week 14 and 52, and endoscopic improvement (EI) between two groups.
Methods
Retrospective cohort study conducted in an Inflammatory Bowel Disease (IBD) unit of a tertiary care hospital. Biologic-naïve patients diagnosed of CD or UC who initiated IFX from 2019 to 2021 (p-TDM group) and from 2016 to 2019 (no-TDM group) were included.
In the p-TDM group, proactive IFX trough levels and antidrug antibodies were measured since week 2 and dose adjustment since week 6 was performed using a previously validated population pharmacokinetic model (Fasanmade et al. Clinical Therapeutics 2011).
In the no-TDM group, dose escalation was performed considering symptoms, blood, or stool results.
CR was defined as Harvey-Bradshaw Index <5 in CD and partial Mayo clinic score <3 in UC. BR was stablished as fecal calprotectin (FCP) <150 mcg/g or c-reactive protein <0.5 mg/dL. EI was defined as lack of ulcerations in CD and Mayo endoscopic score 0-1 in UC. A combined variable to evaluate objective remission (OR) at week 52 was defined as: FCP <150 mcg/g or EI in UC, and EI or radiologic improvement (MRI) in CD.
Moreover, surgery, hospitalization and treatment discontinuation rates were compared between both groups.
Results
30 patients were included in each group: 13 UC, 16 CD and 1 IBD unclassified. There were no statistical differences in baseline characteristic between groups (table 1).
Outcomes at week 14 and 52 are shown in table 2.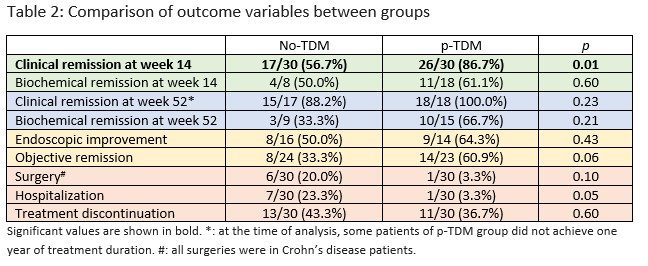
At week 14, CR rate was significantly higher in p-TDM than in no-TDM (86.7% vs 56.7%, p=0.01). Although BR rate at week 14 was numerically higher in p-TDM, it did not achieve statistical significance (61.1% in p-TDM vs 50.0% in no-TDM, p=0.59). However, limitations in blood and stool collection were considerable at this timepoint.
During first year, hospitalizations tend to be fewer in p-TDM group. There were no statistically differences in CR and BR or in EI between groups at week 52. Although OR rate was higher in p-TDM than in no-TDM, it did not reach statistical significance (60.9% vs 33.3%, p=0.06).Conclusion
Proactive TDM during induction phase, especially in naïve patients, could be a useful tool to obtain optimal IFX trough levels and to classify correctly primary failure.


