P730 Anti tumor necrosis factor treatment in Ulcerative Colitis: Real-world data and evaluation of current treatment targets (STRIDE II)
Lundekvam, J.A.(1,2)*;Lie Høivik, M.(1,2);Anisdahl, K.(1,2);Cvancarova Småstuen, M.(3);Medhus, A.W.(1,2);
(1)Oslo University Hospital, Department of Gastroenterology, Oslo, Norway;(2)University of Oslo, Institute of Clinical Medicine, Oslo, Norway;(3)Oslo Metropolitan University, Department of Public Health, Oslo, Norway; Inflammatory Bowel Disease Research Group - Oslo University Hospital
Background
In 2021, the Selection of Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative proposed updated recommendations for treatment targets for ulcerative colitis (UC) patients. The recommendations introduce clinical remission as a short/medium term target, normalisation of CRP and Fecal Calprotectin (FC) as an intermediate target, and advice that change of treatment should be considered if targets are not met. In this single-centre study, we report real-world experience with proactive therapeutic drug monitoring (TDM) strategy for UC patients with first-line anti tumor necrosis factor (TNFi) treatment, and reflect on how implementation of the STRIDE II recommendations might affect clinical practice.
Methods
Adult, biologically naïve UC patients starting TNFi between 2014 and 2021 at Oslo University Hospital were included in a medical chart review study, and data were recorded at three and twelve months. Clinical remission was defined as a patient-recorded outcome (PRO-2) ≤1. Biochemical remission was defined as CRP <5 mg/L and FC < 250 µg/g. Combined remission was defined as clinical and biochemical remission. Target serum drug levels were defined as ≥7.5 mg/L (adalimumab [ADA]) and ≥5 mg/L (infliximab [IFX]). Logistic regression was applied for association analyses.
Results
A total of 141 patients were included. Of these, 60% were male, median age was 25 years. In total, 67% received IFX and 32% received ADA. One third (n=39) patients discontinued TNFi or added a second biologic agent during follow-up and were considered in non-remission, resulting in 102 (72%) persistent patients. Percentages [95% CI] in remission at 3 and 12 months are summarized in Table 1, and for drug-persistent patients in Table 2. Target drug level at 3 months was associated with clinical remission at 12 months (OR 2.97, 95% CI [1.24 – 7.12]), biochemical remission at 12 months (OR 2.64, 95% CI [1.03 – 6.77]) but not with combined remission. In total, 125 dosage adjustments were recorded. Of these, 70 (56%) were classified as adjustments related only to serum drug levels.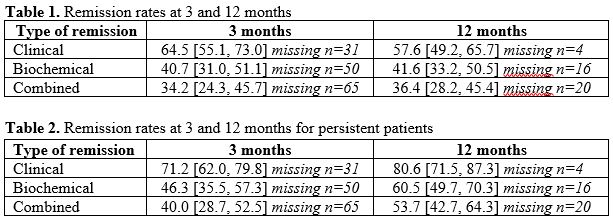
Conclusion
Combined remission rates at 3 and 12 months for persistent patients suggest that 60% and 46% of our cohort should have been considered for a change of treatment according to the STRIDE II recommendations. A majority of dosage adjustments were made proactively, and as target drug levels at 3 months were associated with remission outcomes at 12 months, our data support the use of a proactive TDM strategy.


