P744 Safety of live vaccines in children exposed to biological agents for inflammatory bowel disease (IBD) in utero or during breastfeeding
Chaparro, M.(1)*;García Donday, M.(1);Rubio, S.(2);Calviño Suarez, C.(3); Nuñez Ortiz, A.(4);Figueira, M.(5);Marín Pedrosa, S.(6);Rivero, M.(7); Fernández-Clotet, A.(8);Madero, L.(9);Diz-Lois Palomares, M.T.(10);Pérez-Martínez, I.(11);Ruiz-Cerulla, A.(12);Arroyo, M.(13);Piqueras, M.(14); Suarez Ferrer, C.(15);Aguas, M.(16);Calvo Moya, M.(17);Guerra, I.(18); López Serrano, P.(19);Vázquez Morón, J.M.(20);Arias García, L.(21);Casanova, M.J.(1);Huguet, J.M.(22);Valldosera Gomis, G.(23);Zúñiga de Mora-Figueroa, B.(24);Armesto, R.(25);Martínez Montiel, P.(26);Rodríguez-Lago, I.(27);Sendra Rumbeu, P.(28);Camargo Camero, R.(29);Hervías Cruz, D.(30);Molina Arriero, G.(31);Barreiro-de Acosta, M.(3);Acosta, D.(1);Brenes, Y.(1);Hermida, S.(1);Parra, P.(1);Garre, A.(1);Gisbert, J.P.(1);
(1)Hospital Universitario de La Princesa- IIS-Princesa- Universidad Autónoma de Madrid UAM- and CIBEREHD, Gastroenterology Unit, Madrid, Spain;(2)Hospital Universitario de Navarra, Gastroenterology Unit, Pamplona, Spain;(3)Complexo Hospitalario Universitario de Santiago de Compostela, Gastroenterology Unit, Santiago de Compostela, Spain;(4)Hospital Universitario Virgen del Rocío, Gastroenterology Unit, Sevilla, Spain;(5)Xerencia Xestión Integrada de Vigo- SERGAS. Grupo de Investigación de Patología Digestiva. IS Galicia Sur. SERGAS UVIGO, Gastroenterology Unit, Vigo, Spain;(6)Hospital Universitario Reina Sofía, Gastroenterology Unit, Córdoba, Spain;(7)Hospital Universitario Marqués de Valdecilla e IDIVAL, Gastroenterology Unit, Santander, Spain;(8)Hospital Universitario Clìnic i Provincial, Gastroenterology Unit, Barcelona, Spain;(9)Hospital General Universitario de Alicante, Gastroenterology Unit, Alicante, Spain;(10)Complexo Hospitalario Universitario de A Coruña, Gastroenterology Unit, A Coruña, Spain;(11)Hospital Universitario Central de Asturias and ISPA, Gastroenterology Unit, Oviedo, Spain;(12)Hospital Universitario de Bellvitge, Gastroenterology Unit, Barcelona, Spain;(13)Hospital Clínico Universitario Lozano Blesa- IIS Aragón and CIBEREHD, Gastroenterology Unit, Zaragoza, Spain;(14)Consorci Sanitari de Terrassa CST, Gastroenterology Unit, Barcelona, Spain;(15)Hospital Universitario La Paz, Gastroenterology Unit, Madrid, Spain;(16)Hospital Universitario y Politécnico La Fe and CIBEREHD, Gastroenterology Unit, Valencia, Spain;(17)Hospital Universitario Puerta de Hierro Majadahonda, Gastroenterology Unit, Madrid, Spain;(18)Hospital Universitario de Fuenlabrada and IdiPAZ, Gastroenterology Unit, Madrid, Spain;(19)Hospital Universitario Fundación Alcorcón, Gastroenterology Unit, Madrid, Spain;(20)Hospital Universitario Juan Ramón Jiménez, Gastroenterology Unit, Huelva, Spain;(21)Hospital Universitario de Burgos, Gastroenterology Unit, Burgos, Spain;(22)Hospital General Universitario de Valencia, Gastroenterology Unit, Valencia, Spain;(23)Hospital Universitario Joan XXIII, Gastroenterology Unit, Tarragona, Spain;(24)Hospital Clínico Universitario San Cecilio, Gastroenterology Unit, Granada, Spain;(25)Complexo Hospitalario Universitario de Ourense, Gastroenterology Unit, Ourense, Spain;(26)Hospital Universitario 12 de Octubre, Gastroenterology Unit, Madrid, Spain;(27)Hospital Universitario de Galdakao- Instituto de Investigación Sanitaria Biocruces Bizkaia, Gastroenterology Unit, Galdakao, Spain;(28)Hospital Universitario Son Espases, Gastroenterology Unit, Palma de Mallorca, Spain;(29)Hospital Universitario Virgen de La Victoria, Gastroenterology Unit, Málaga, Spain;(30)Hospital General Universitario de Ciudad Real, Gastroenterology Unit, Ciudad Real, Spain;(31)Complejo Hospitalario Universitario de Ferrol, Gastroenterology Unit, Ferrol, Spain; on behalf of DUMBO study group of GETECCU
Background
Biological drugs used for IBD are detected in breast milk at a concentration below 10% of the maternal serum concentration and have therefore been considered as permitted drugs. However, warnings have recently been published regarding vaccination with live agents in children whose mothers receive infliximab during breastfeeding.
Aim: To assess the risk of serious adverse events related to the administration of live vaccines in children exposed to biological drugs in utero or whose mothers were receiving biological agents during breastfeeding.
Methods
Children born to IBD mothers from DUMBO registry of GETECCU were included. DUMBO is a prospective, observational and multicentre registry, which enrolls pregnant women with IBD over 5 years in 70 centres in Spain. Data on treatment during gestation, type of lactation, breastfeeding, end-date of breastfeeding, maternal treatments during breastfeeding and serious adverse events in children from birth are being prospectively included contacting with the mothers every-3-months. Following the Spanish immunization calendar, rotavirus vaccine is (voluntarily) administered at 2, 4 and 6 (3rd dose only with Rotateq®) months; measles, mumps and rubella (MMR) at 12 months and 3-4 years of age; and varicella at 15 months and 3-4 years of age.
Results
526 newborns were included in the registry at the time of data analysis. A total of 205 (39%) had been exposed to biologics during pregnancy or breastfeeding (table 1): 80 (42%) during pregnancy, 7 (2%) during breastfeeding, and 109 (57%) during both. Newborn’s demographics and exposure to drugs during breastfeeding are summarized in table 2. Mean follow-up was 12 months; proportion of children breastfed during follow-up is shown in table 3a. The percentage of children who had been vaccinated according to the recommended schedule was above 95% at all visits (table 3b). Live vaccines administered to children exposed in utero to biologics during the 3rd trimester of gestation are shown in table 4a. From birth, 71% of infants were breastfed (52% exclusively breastfed). Live vaccines administered to children breastfed at least until month 6, until month 12 and until month 15 are summarized in table 4b. No serious adverse event related to live vaccine was reported in our cohort.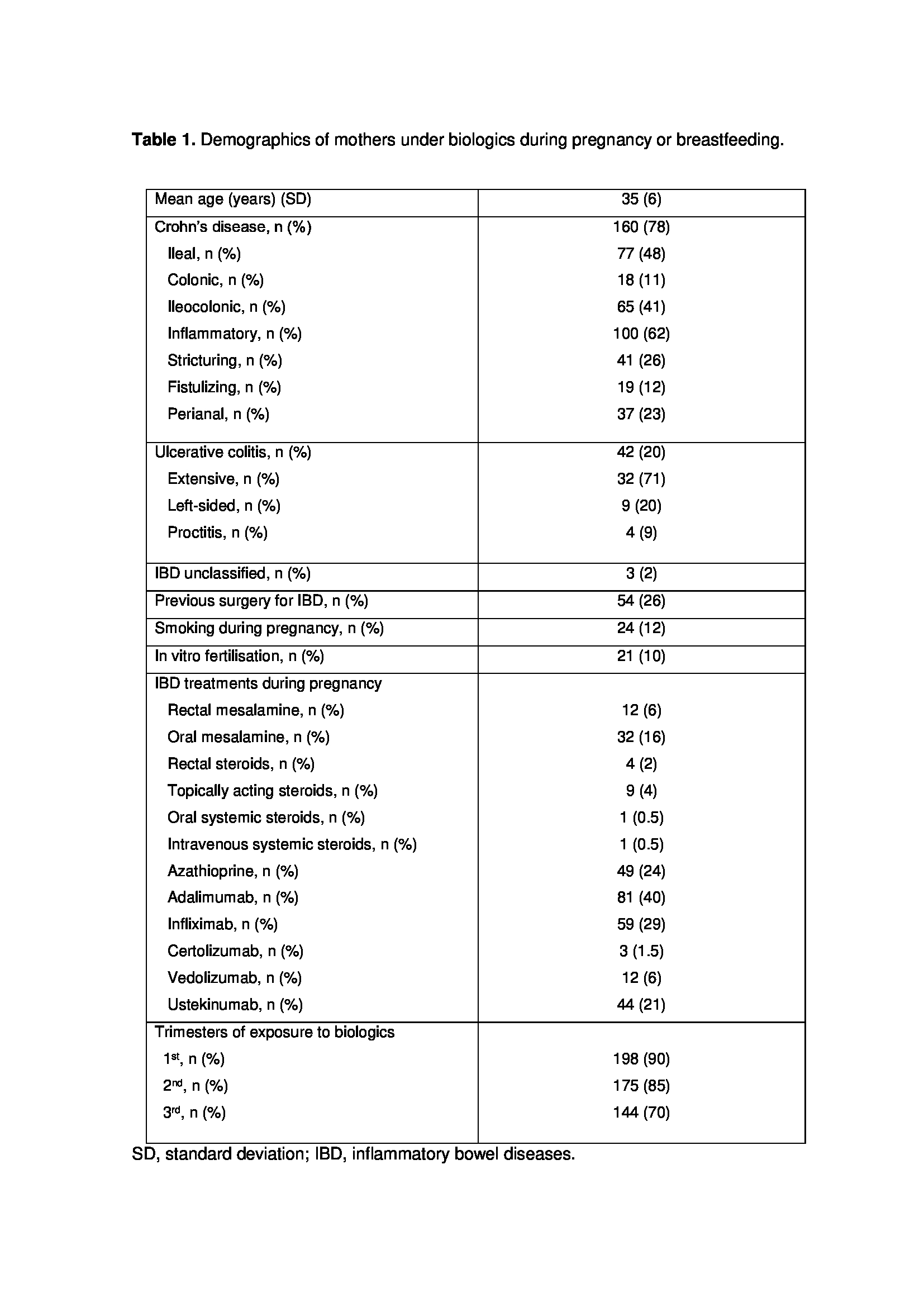


.jpeg)
Conclusion
Administration of live virus vaccines from 12 months of age in children born to IBD mothers and exposed to biological drugs in utero or during breastfeeding seems safe and should not be recommended against vaccination or breastfeeding. Rotavirus vaccine (under 6 months of age) appears to be equally safe in these children.


