P752 Predictors of time to relapse upon ozanimod withdrawal in patients with moderately to severely active ulcerative colitis
Vermeire, S.(1)*;Abraham, B.(2);Bressler, B.(3);Reinisch, W.(4);Axelrad, J.E.(5);Jain, A.(6);Memaj, A.(6);Akukwe, L.(6);Liu, W.J.(6);Osterman, M.T.(6);Canavan, J.B.(6);Sands, B.E.(7);
(1)University of Leuven, Chronic Diseases and Metabolism, Leuven, Belgium;(2)Houston Methodist-Weill Cornell Medical, Gastroenterology, Cornell, United States;(3)University of British Columbia, Medicine, Vancouver, Canada;(4)Medical University of Vienna, Gastroenterology and Hepatology, Vienna, Austria;(5)NYU Grossman School of Medicine, Medicine, New York, United States;(6)Bristol Myers Squibb, Clinical Research, Princeton, United States;(7)Icahn School of Medicine at Mount Sinai, Gastroenterology, New York, United States;
Background
Ozanimod (OZA) is approved in the EU, US, and other countries for the treatment of moderately to severely active ulcerative colitis (UC) in adults based on the phase 3 True North (TN) trial. Data on time to disease relapse in patients (pts) who withdrew OZA treatment in TN were previously reported (Sands BE et al. Am J Gastroenterol. 2022;17(suppl):S506-7). The objective of this post hoc analysis was to identify predictive and prognostic variables of time to disease relapse in the TN maintenance period (MP).
Methods
Pts in TN were randomised to once-daily oral OZA 0.92 mg or placebo (PBO) in a double-blind manner or to open-label OZA for a 10-week induction period. Those with clinical response to OZA at Week 10 were then rerandomised to continue OZA (OZA/OZA) or switch to PBO (OZA/PBO) in the MP. Disease relapse was defined as an increase in partial Mayo score of ≥2 points from Week 10, absolute partial Mayo score ≥4, Mayo endoscopy subscore (MES) ≥2, and exclusion of other causes of increased disease activity unrelated to UC. Time to disease relapse was estimated using the Kaplan-Meier method in both MP groups. The saturated Cox proportional-hazards model for assessing predictors of time to disease relapse included demographics, disease characteristics, prior medications, and laboratory and efficacy variables at the end of the induction period. Insignificant terms at α=0.05 were removed via backward elimination.
Results
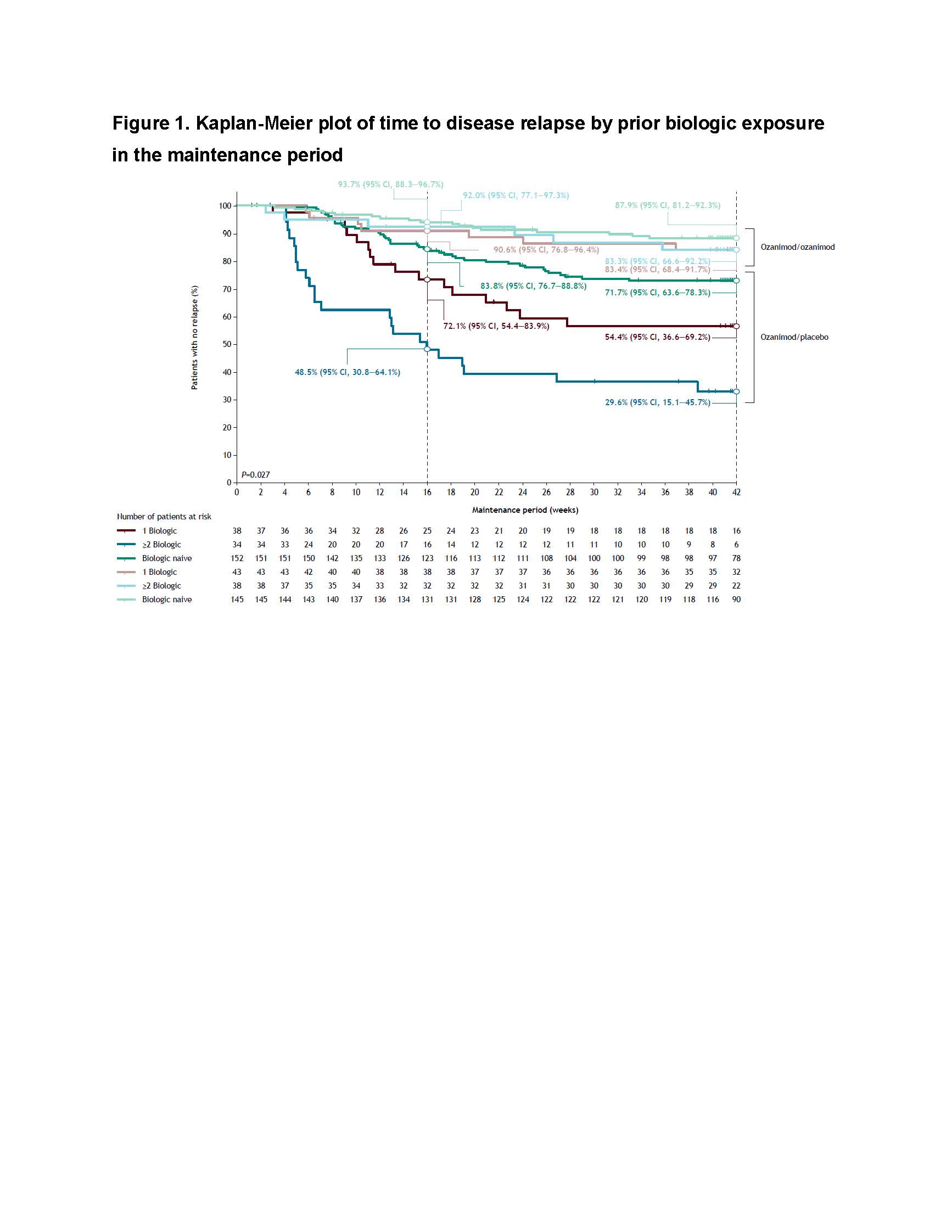
The OZA/OZA arm (n=230) had a slightly higher proportion of pts with baseline severe endoscopic disease than the OZA/PBO arm (n=227). Otherwise, baseline and Week 10 characteristics were balanced across MP arms (Tables 1 and 2). In multivariable analyses, corticosteroid use at Week 10 was prognostic for time to relapse (P<0.0001; no interaction with individual treatment arms). MES >1 and clinical response but not remission at Week 10 were also assessed as prognostic variables, but these did not achieve statistical significance at α=0.05 (P=0.071 and P=0.171, respectively). Relapse rates were low and similar regardless of prior biologic exposure in the OZA/OZA arm. Prior biologic exposure was an independent predictor of time to relapse after withdrawal of OZA in the OZA/PBO arm (P=0.027) (Figure 1). For OZA/PBO pts, Week 16 Kaplan-Meier estimates and 95% CI of no relapse were 83.8% (76.7–88.8%), 72.1% (54.4–83.9%), and 48.5% (30.8–64.1%) for the biologic-naive, 1 failed biologic, and ≥2 failed biologics groups, respectively.

Conclusion
Increasing numbers of prior biologics were predictive of increased rates of disease relapse in pts who discontinued OZA. Pts with UC, especially those with prior biologic failures, should minimise OZA discontinuation to mitigate the risk of disease relapse.


