P758 Impact of co-medication on rates of endoscopic remission: post-hoc analysis of randomised non-inferiority trial of 1600 mg versus 400 mg tablets of mesalazine for the treatment of mild-to-moderate ulcerative colitis
Safroneeva, E.(1)*;Laoun, R.(1);
(1)Tillotts Pharma AG, Medical Affairs, Rheinfelden, Switzerland;
Background
Some studies reported that anti-TNF treatment in biologic-naïve elderly onset inflammatory bowel disease patients may be less effective than in non-elderly onset patients (Amano et al. Scientic Reports. 2022 12:5324). We assessed known factors as well as presence of co-morbidities and polypharmacy associated with endoscopic remission in mild-to-moderate (Mayo clinic score ≥5) adult ulcerative colitis (UC) patients treated with mesalazine.
Methods
In a post-hoc analysis of data from a phase 3 non-inferiority trial ( D’Haens et al. Aliment Pharmacol Ther.2017;46:292–302)we examined the strength of association between number of co-medications and comorbidities by calculating Spearman’s rho. We used multivariable logistic regression modeling to determine the associations between endoscopic remission as outcome (yes vs. no; defined as either Mayo endoscopic subscore of 0 or ≤1 according to blinded central reading) and independent variables (disease duration, Mayo endoscopic score at baseline, leucocyte concentration at baseline, Robarts Histopathologic Index at baseline, age [≤60 vs. >60 years of age], gender, comedications at the time of the trial [none, ≤2, ≥3], comorbidities [none, 1-2, 3-5, >5], body mass index, disease extent prior to the trial, smoking status [current, ex, and never] at week 8 and 38. Collinear variables were examined in separate models. P-value of <0.05 was considered to be statisically significant.
Results
We analyzed data on 774 patients (330 female, 43.5±14.3 [SD] years of age, median disease duration of 3.00 years [IQR 0.56; 7.88], 175 and 92 persons with 1-2 and ≥3 comedications, respectively) and 679 patients (289 female, 43.7±14.5 [SD] years of age, median disease duration of 2.99 years [0.57; 7.62]; 150 and 85 persons with 1-2 and ≥3 comedications, respectively) that had colonoscopy/sigmoidoscopy at 8 weeks of double-blind induction and 38 weeks open-label maintenance phases of the study, respectively. We observed a moderate association between number of comorbidities and comedications (week 8: Spearman rho=0.545, P-value of < 0.001; week 38: Spearman rho=0.512, P-value < 0.001). Patients with baseline Mayo clinic endoscopic score of 3 and increased baseline leucocyte concentration, were less likely to be in endoscopic remission irrespective of endoscopic remission definition and time post treatment (Table 1). In addition, patients with ≥3 co-medications were less likely to be in endoscopic remission of 1 at week 8 and 38 and in endoscopic remission of ≤1 at week 38 (Table 1).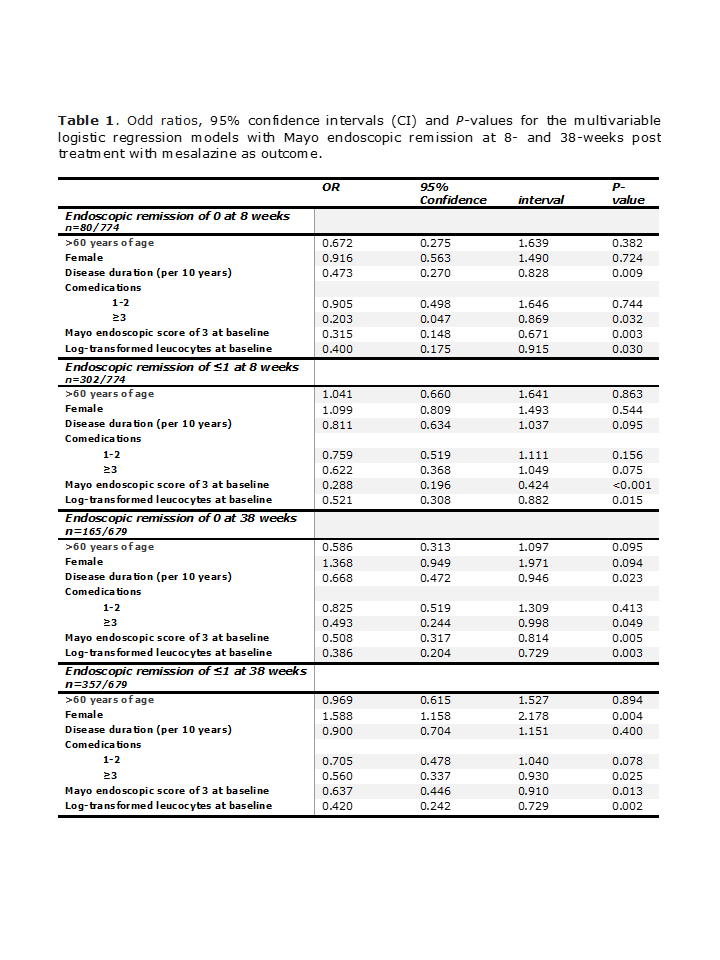
Conclusion
The increased number of co-medications rather than the actual age may be responsible for decreased effectiveness of medications, including mesalazine, in elderly UC patients highlighting the importance of healthy aging.


