P781 Treatment outcome of tofacitinib dose reduction to 5 mg BID vs remaining on 10 mg BID in patients with Ulcerative Colitis who were in stable remission on 10 mg BID: Updated 30-month data from the double-blind, randomised RIVETING study
Rubin, D.T.(1);Panés, J.(2);Torres, J.(3,4,5);Kobayashi, T.(6);Su, C.(7);Lawendy, N.(7);Modesto, I.(8);Segovia, M.(7,9);Gardiner, S.(8);Wang, W.(7);Vermeire, S.(10)*;
(1)-, University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago- IL, United States;(2)Formerly Department of Gastroenterology, Hospital Clínic de Barcelona- IDIBAPS- CIBERehd, Barcelona, Spain;(3)Gastroenterology Division, Hospital Beatriz Ângelo, Loures, Portugal;(4)Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal;(5)Division of Gastroenterology, Hospital da Luz, Lisbon, Portugal;(6)Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Toyko, Japan;(7)-, Pfizer Inc, Collegeville- PA, United States;(8)-, Pfizer Inc, New York- NY, United States;(9)-, Rutgers University, New Brunswick- NJ, United States;(10)Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium;
Background
Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of ulcerative colitis. The long-term, Phase 3b/4 RIVETING study (NCT03281304) assessed the efficacy and safety of tofacitinib dose reduction from tofacitinib 10 mg twice daily (BID) maintenance therapy to 5 mg BID in patients (pts) in stable remission.1 We present a final analysis of the RIVETING study after ≥30 months of treatment.
Methods
RIVETING was a double-blind, randomised, parallel-group study with a 42-month (M) duration. Eligible pts had received tofacitinib 10 mg BID for ≥2 consecutive years in an open-label, long‑term extension study (NCT01470612), had been in stable remission for ≥6 months and corticosteroid-free for ≥4 weeks prior to baseline. The primary efficacy endpoint was remission at M6 based on modified Mayo (mMayo) score (endoscopic and stool frequency subscores of ≤1 and rectal bleeding subscore of 0).1 RIVETING was terminated (primary objective met) when all pts had passed M30 (or discontinued); some pts had already completed all visits up to M42. Here, we assess efficacy at M30 and safety throughout.
Results
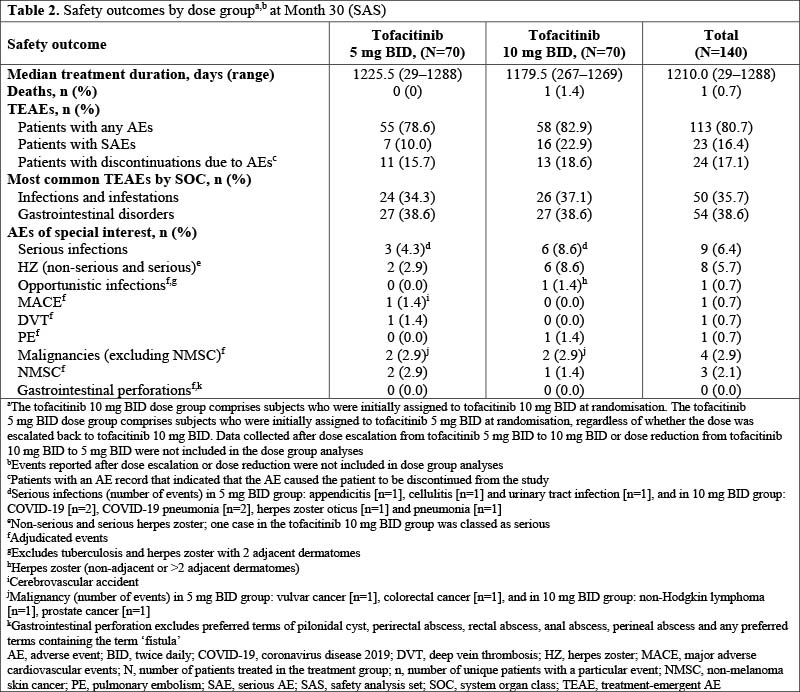
Overall, 140 pts were randomised (1:1) to tofacitinib 5 or 10 mg BID; 50.0% and 62.9% were in remission based on mMayo score at M30 in the 5 and 10 mg BID dose groups, respectively, with consistent findings observed for other secondary efficacy endpoints (Table 1). At M30, observed differences for mMayo remission between tofacitinib 10 and 5 mg BID were generally greater in the subgroup with a baseline endoscopic subscore of 1 vs 0 and in the subgroup with vs without prior tumour necrosis factor inhibitor (TNFi) failure (Table 1). The percentage of pts who experienced loss of remission by M30, as estimated from Kaplan-Meier curves, was numerically higher in the 5 vs the 10 mg BID dose group (28.1%; 95% confidence interval [CI], 17.68–39.49 vs 21.7%; 95% CI, 12.21–32.95, respectively). Table 2 shows adverse events of special interest, by dose group. One death occurred due to fatal coronavirus disease 2019 pneumonia (10 mg BID).
Conclusion
These long-term data showed that most pts in stable remission on tofacitinib 10 mg BID maintenance therapy maintained mMayo score remission through M30 after dose reduction to 5 mg BID. Dose group difference in remission at M30 was consistent with that at M6.1 Differences in remission between the tofacitinib 5 and 10 mg BID groups were greater in subgroups with an endoscopic subscore of 1 vs 0 and in subgroups with vs without prior TNFi failure. Overall, safety findings were consistent with tofacitinib’s known safety profile; incidence of serious infections and herpes zoster was numerically higher in the tofacitinib 10 vs 5 mg BID group.
1. Vermeire S et al. J Crohns Colitis 2021;15:1130-41.


