P783 Symptom Improvement of ulceRative colitis after an Injection dose of Ustekinumab in Japanese clinical practice, measured using patient-reported outcomes (SIRIUS) study, Interim Analysis
Matsuoka, K.(1)*;Katsumasa, N.(2);Murata, Y.(2);Hisamatsu, T.(3);
(1)Toho University Sakura Medical Center, Division of Gastroenterology and Hepatology- Department of Internal Medicine, Sakura-city- Chiba, Japan;(2)Janssen Pharmaceutical K.K., Medical Affairs Division, Chiyoda-ku- Tokyo, Japan;(3)Kyorin University School of Medicine, Department of Gastroenterology and Hepatology, Mitaka-city- Tokyo, Japan; SIRIUS study group
Background
Ustekinumab (UST) is treated to moderate to severe adult ulcerative colitis (UC) patients. We conducted the SIRIUS study (Symptom Improvement of ulceRative colitis after an Injection dose of UStekinumab in Japanese clinical practice, measured using patient-reported outcomes (PRO)) to record daily symptoms for 8 weeks after the introduction of intravenous ustekinumab in moderate to severe UC patients, which aimed to reveal early response patterns of symptoms during the induction of remission period with ustekinumab, and to explore symptoms suitable for monitoring and predicting treatment effect. This interim analysis focuses on stool frequency (SF) and rectal bleeding (RB) scores in the PRO-2 during the 8-week induction period.
Methods
This single-arm prospective observational study conducted in 23 centers in Japan aimed to recruit 140 patients with moderate-to-severe UC between July 2021 and July 2022. RB score, SF score, abdominal pain, nocturnal diarrhea, tenesmus, and patient perception of improvement were recorded daily using a smartphone app. To be eligible for the study, participants must have a documented diagnosis of UC, be ≥16 years of age, have UC (partial Mayo score of 5~9), have had an inadequate response or intolerance to prior UC therapies, and be able to understand and complete PRO instruments using a smartphone. Patients who had prior exposure to UST, history of extensive colectomy, or were hospitalized when UST was started were excluded.
Results
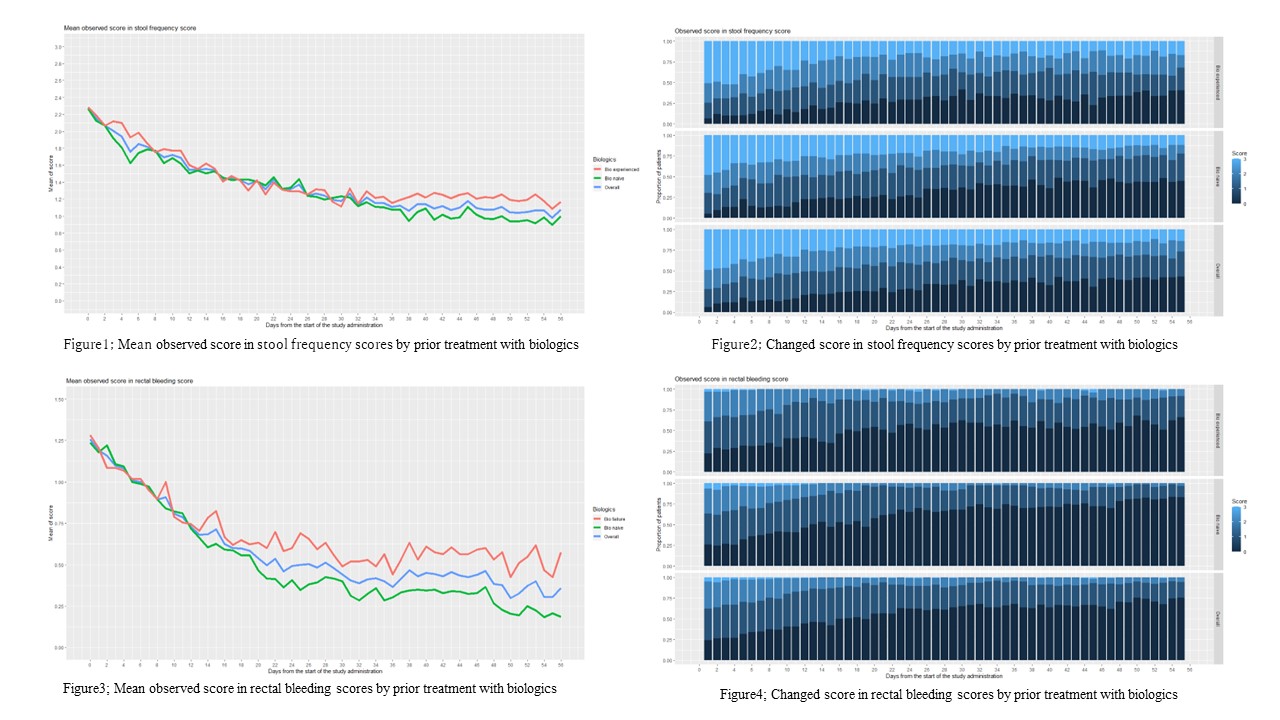
In this analysis,137 patients were included. Seventy-four (54.4%) patients were male and the median age was 35 years. Seventy-six (55.9%) patients were Bio-naïve. This high proportion of Bio-naïve patients reflects the fact that UST can be used for Bio-naive patients without restrictions in the Japanese health insurance. At weeks 2 and 4, 56 (41.2%) and 57 (41.9%) patients attended outpatient clinics, respectively. The data collection rate of the PROs was 95%. Mean SF and RB scores were demonstrated stratified by prior treatment with Bio (Figure 1&3). Significant decreases in mean SF and RB scores were observed within 1 week in both Bio-naïve and -experienced patients. the time-course change in SF was similar between Bio-naïve and -experienced patients, but the decrease in RB scores was greater in the Bio-naïve patients than the Bio-experienced patients.
Conclusion
A daily PRO-based analysis was conducted in the Sirius study to monitor symptoms during the first 8 weeks after the introduction of UST in patients with moderate-to-severe UC. This interim analysis revealed that SF and RB scores started to decrease within 1 week, demonstrating a rapid symptomatic response to UST. The analysis of the other PROs and predictors of efficacy will be performed in further studies.