P806 Perianal Crohn’s Disease (pCD): The outcome of 2nd line and 3rd line biologics in perianal Crohn’s disease
Shani, U.(1)*;Ben Shabat, N.(1);Klang, E.(2);Ungar, B.(3);Ben-Horin, S.(3);Kopylov, U.(3);
(1)Sheba Medical Center, Department of Internal Medicine B, Ramat Gan, Israel;(2)Sheba Medical Center, ARC innovation center, Ramat Gan, Israel;(3)Sheba Medical Center, Department of Gastroenterology, Ramat Gan, Israel;
Background
Treating perianal Crohn’s disease (pCD) is challenging with at least 60% of the patients not responding or relapsing after a year of 1st line biological therapy. Effectiveness data on 2nd and 3rd line in pCD is still quite limited. The aim of this study was to describe the effectiveness of 2nd and 3rd line biological therapy in pCD.
Methods
A retrospective cohort study of patients with pCD from a large tertiary center registry. We included all patients with pCD that were treated with >1 biologic while anti-TNF was the first one used. Primary outcome was defined as clinical failure of 2nd or 3rd line treatment (as defined by the clinician) or radiological evidence for disease progression by MRI or transrectal ultrasonography (TRUS). Secondary outcomes were relapse of perianal fistula or abscess.
Results
Registry included 893 patients with pCD who are being followed up routinely in a dedicated IBD clinic; 643 patients received 1 biological were excluded. Final cohort included 245 patients treated with ≥2 biologics.
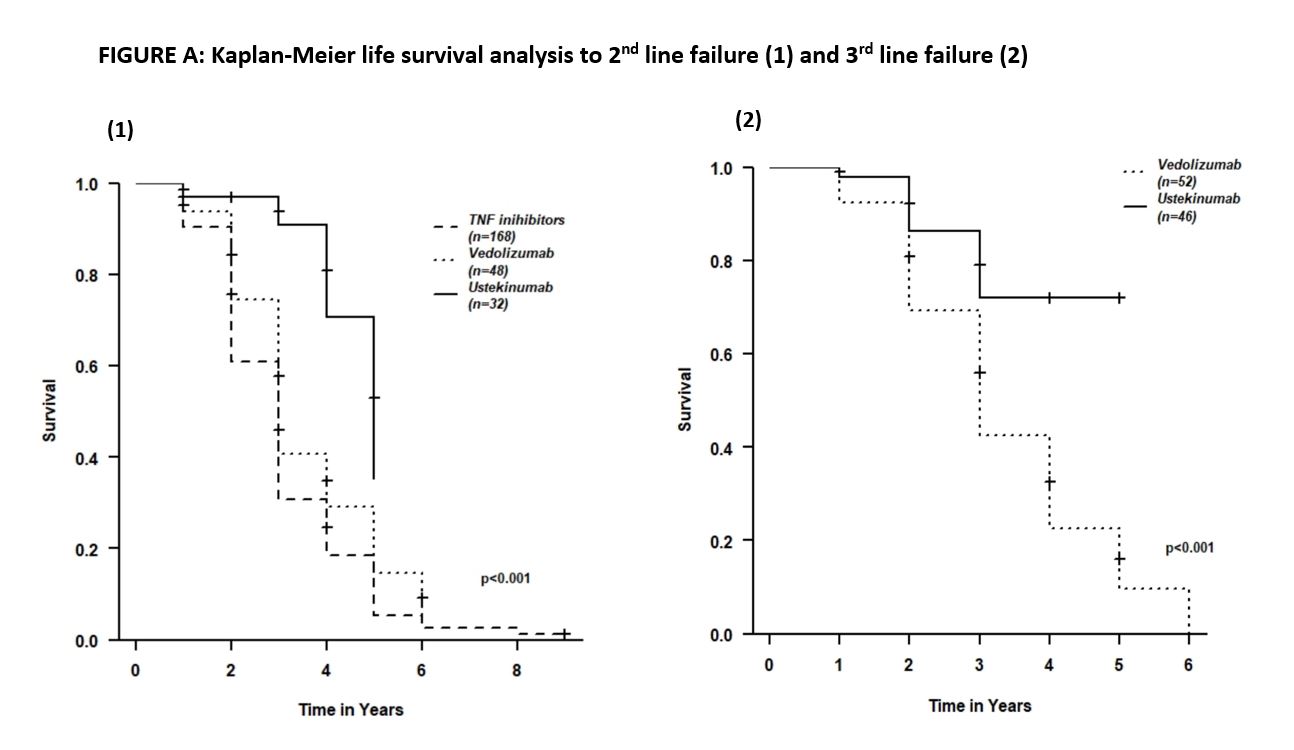
In 2nd line, 40/245 patients (16%) treated with infliximab (IFX), 128/245 (52%) with adalimumab (ADA), 48/245 (20%) with vedolizumab (VDZ) and 32/245 (13%) with ustekinumab (UST). 7/32 patients (22%) treated with UST failed the treatment, compared to 32/40 (80%), 101/128 (79%) , 37/48 (77%) patients treated with IFX, ADA and VDZ, respectively [Hazard Ratio(HR) 0.27 (0.1-0.69)]. Median time of 2nd line treatment with UST, anti-TNFs and VDZb was 2.5 (IQR=1-3[uk1] ), 3 (IQR=1-5) and 3 (IQR=1-4) years, respectively. Higher response rate in 2nd line therapy was associated with longer response duration to 1st line [OR (1.11-1.55), p<0.001)].
99/245 patients (40%) required 3rd line treatment (VDZ - 52/99 , UST - 47/99 (48%)) patients). 8/47 patients (17%) under UST therapy experienced clinical failure, compared to 41/52 (78.8%) patients treated with VDZ [HR 0.32 (0.12-0.68)]. Median duration (years) of third line treatment with both UST and VDZ was 3 years (IQR=3-1).
Figure A shows Kaplan-Meier survival analysis to 2nd line and 3rd line failure.
Conclusion
2nd and 3rd line biological therapies may be efficacious in pCD patients. Ustekinumab appears to be effective as a 2nd or 3rd line treatment. Longer duration of treatment is associated with a higher likelihood of response to 2nd line treatment.