P911 The changes in the faecal microbiome and metabolome in Crohn's disease after treatment with anti-TNFα antibodies are of secondary origin
Hurych, J.(1,2)*;Mascellani Bergo, A.(3);Lerchová, T.(2);Hlináková, L.(1);Kubát , M.(2);Malcová, H.(4);Cebecauerová, D.(4);Schwarz, J.(5);Karásková, E.(6);Hecht, T.(7);Vyhnánek, R.(7);Ťoukálková, L.(8);Dotlačil, V.(9);Kobrová, K.(10);Čížková, A.(11);Horváth, R.(4);Bronsky, J.(2);Havlík, J.(3);Hradský, O.(2);Cinek, O.(2);
(1)Charles University- Second Faculty of Medicine, Department of Medical Microbiology, Prague, Czech Republic;(2)Charles University- Second Faculty of Medicine, Department of Paediatrics, Prague, Czech Republic;(3)Czech Univesity of Life Sciences-Faculty of Agrobiology- Food and Natural Resources, Department of Food Science, Prague, Czech Republic;(4)Motol University Hospital, Department of Pediatric and Adult Rheumatology, Prague, Czech Republic;(5)Charles University- Faculty of Medicine in Pilsen, Department of Paediatrics, Pilsen, Czech Republic;(6)Palacky University Olomouc- Faculty of Medicine-, Department of Paediatrics, Olomouc, Czech Republic;(7)Charles University- First Faculty of Medicine, Department of Paediatrics, Prague, Czech Republic;(8)Tomas Bata Hospital, Department of Paediatrics, Zlin, Czech Republic;(9)Charles University- Second Faculty of Medicine, Department of Paediatric Surgery, Prague, Czech Republic;(10)Masaryk Hospital, Department of Paediatrics, Usti nad Labem, Czech Republic;(11)Synlab Czech- Inc, Sampling station Prague 7, Prague, Czech Republic;
Background
Treatment by anti-TNFα antibodies (aTNFα) is known to change the dysbiotic faecal bacteriome in Crohn's disease (CD). However, whether the change is caused by aTNFα itself or by improving the gut inflammation status is unknown. Therefore, we aimed to identify and compare bacterial taxa and faecal metabolite changes upon aTNFα between children with CD and juvenile idiopathic arthritis (JIA), a disease very rarely affecting the gut.
Methods
Faecal samples were analysed for bacteriome by massively parallel sequencing of the V4 region of the 16S rRNA and for faecal metabolome by 1H nuclear magnetic resonance. Gut inflammation status was assessed by faecal calprotectin levels.
Results
In total, 530 stool samples from 121 children (CD 54, JIA 18, healthy 49) from six different hospitals in Czechia were collected. In CD patients, 37 progressed to the need for aTNFα therapy, whereas 17 remained on other therapeutic modalities; in JIA, 14 received aTNFα, whereas four remained on other treatments. In JIA, calprotectin levels were not different from controls (P=0.16), and lower than in CD (~50 fold, P<10-16). Among CD, when aTNFα therapy was administered, their calprotectin levels decreased by a mean of 59% as compared to the period off-therapy (P=1.40 x 10-5). Regarding the microbiome and metabolome changes, five bacterial genera and four metabolites significantly changed their abundance/concentration upon aTNFα administration in CD but not in JIA. Within the bacteriome, Blautia, Ruminococcus, Intestinibacter and Erysipelatoclostridium (all phylum Firmicutes) increased, whereas Allistipes (phylum Bacteroidota) decreased. Among metabolites, glucose and glycerol increased, whereas isoleucine and uracil decreased. The analysis of bacteriome composition revealed dysbiosis in CD, with a shift away from Proteobacteria towards Firmicutes and Actinobacteria after aTNFα.

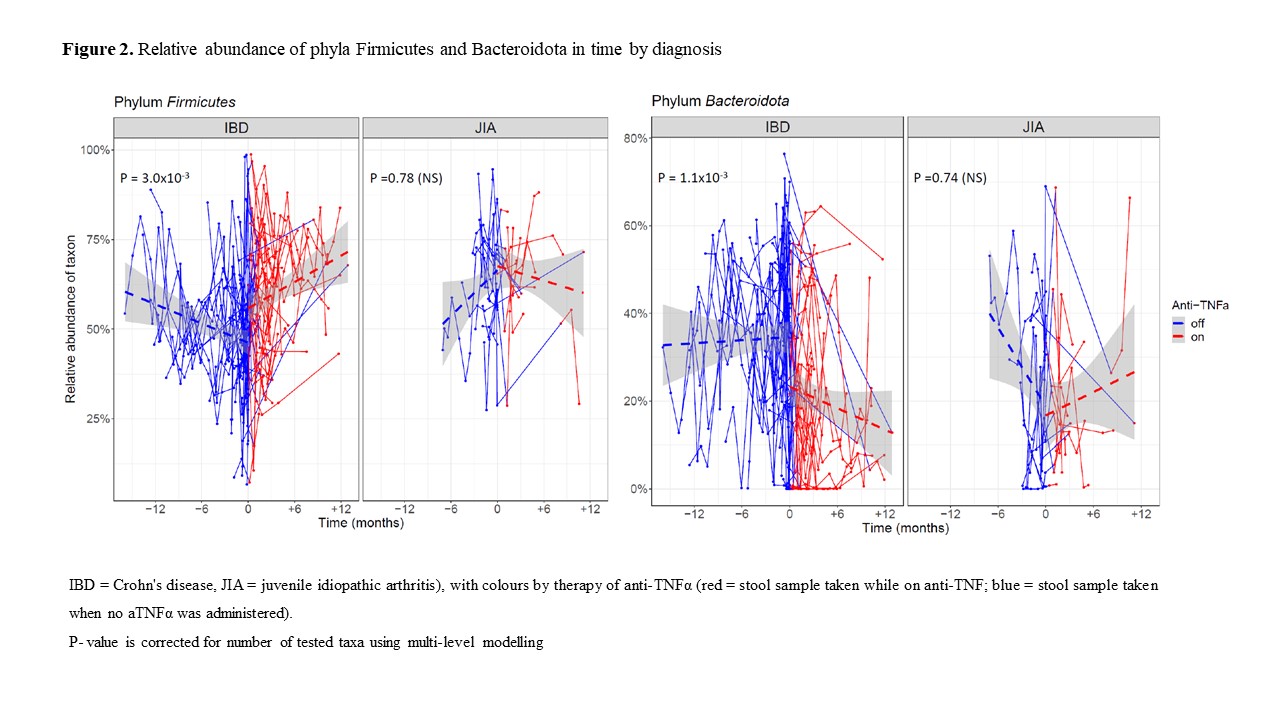
Conclusion
Our findings suggest that aTNFα therapy changes the dysbiotic microbiome in CD towards healthier composition. This is supported by relatively comprehensive longitudinal stool sampling in a multicentric study. Most importantly, the observed bacteriome changes upon treatment with TNFα blockers are of secondary origin, as they occur only in CD patients, whose gut inflammation status improves after the treatment, and no effects are observed in the control group of JIA.
- Posted in: Poster Presentations: Microbiology 2023


